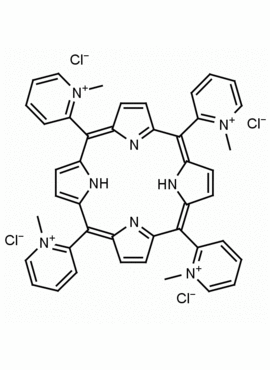
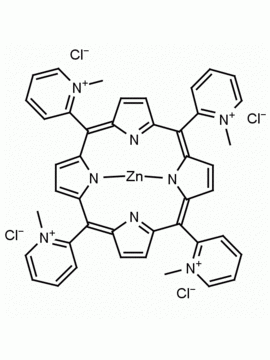
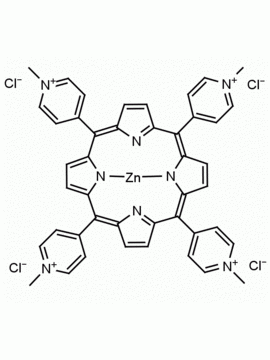
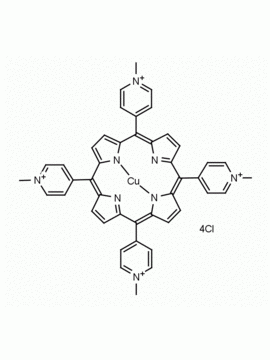
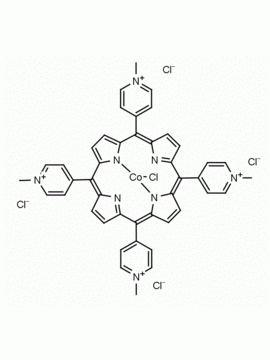
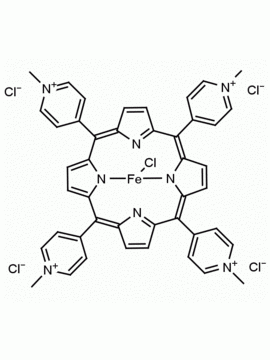
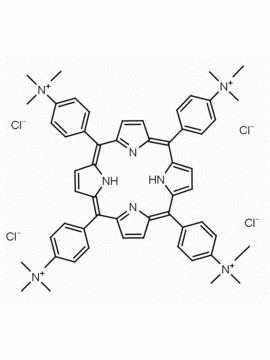
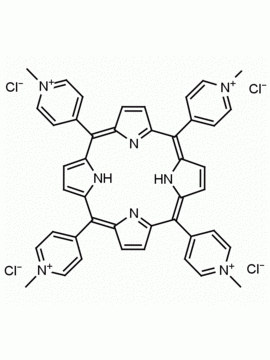
meso-Tetra (N-methyl-2-pyridyl) porphine tetrachloride CAS: 129051-18-9 MDL: MFCD06411460
Molecular weight: 820.638 g/mol
Molecular Formula: C44H38Cl4N8
CAS Number: 129051-18-9
Storage: Store at room temperature, protect from light.
Synonyms: 2,2′,2”,2”’-(5,10
Fields of Interest: Water Soluble Porphyrins, G-Quadruplex
Background: meso-Tetra (N-methyl-2-pyridyl) porphine tetrachloride is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals. meso-Tetra (N-methyl-2-pyridyl) porphine tetrachloride is used as a control in G-quadruplex studies since it does not bind to G-quadruplexes.1-6
References:
1.) David, et al. G-quadruplexes as novel cis-elements controlling transcription during embryonic development. Nucleic Acids Research, Volume 44, Issue 9, 19 May 2016, Pages 4163–4173, https://doi.org/10.1093/nar/gkw011
2.) Alonso, et al. A rapid and sensitive high-throughput screening method to identify compounds targeting protein–nucleic acids interactions. Nucleic Acids Research, Volume 43, Issue 8, 30 April 2015, Page e52, https://doi.org/10.1093/nar/gkv069
3.) Ravichandran, et al. Genome-wide analysis of regulatory G-quadruplexes affecting gene expression in human cytomegalovirus. PLOS Pathogens, Published: September 28, 2018 https://doi.org/10.1371/journal.ppat.1007334
4.) Wu, et al. DDX5 helicase resolves G-quadruplex and is involved in MYC gene transcriptional activation. PNAS. September 23, 2019, 116 (41) 20453-20461. https://doi.org/10.1073/pnas.1909047116
5.) Faudale, et al. Photoactivated cationic alkyl-substituted porphyrin binding to g4-RNA in the 5′-UTR of KRAS oncogene represses translation. Chem. Commun., 2012,48, 874-876. https://doi.org/10.1039/C1CC15850C
6.) Shi, et al. Quadruplex-Interactive Agents as Telomerase Inhibitors: Synthesis of Porphyrins and Structure−Activity Relationship for the Inhibition of Telomerase. J. Med. Chem. 2001, 44, 26, 4509–4523. https://doi.org/10.1021/jm010246u