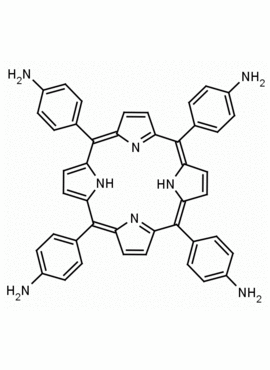
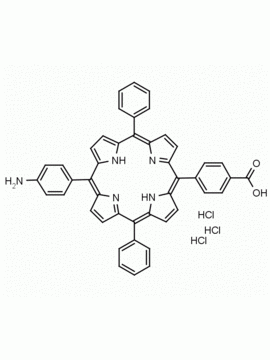
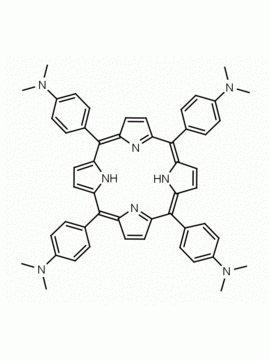
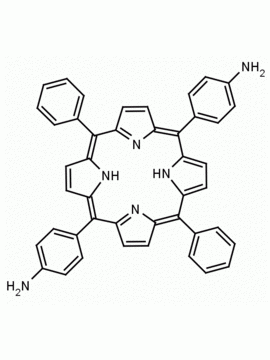
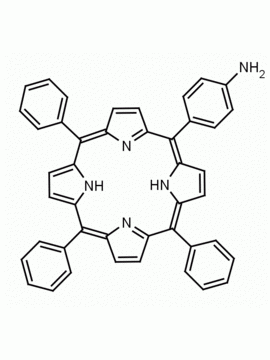
meso-Tetra (4-aminophenyl) Porphine CAS: 22112-84-1 MDL: MFCD00191497
SDS
Molecular weight: 674.81 g/mol
Molecular Formula: C44H34N8
CAS Number: 22112-84-1
Storage: Store at room temperature, protect from light
Synonyms: TPAPP, Tetrakis(4-aminophenyl)porphyrin,meso-Tetra(p-aminophenyl)porphyrin,5,10,15,20-(tetra-4-aminophenyl)porphyrin,5,10,15,20-Tetrakis(4-aminophenyl)porphyrin,5,10,15,20-TETRAKIS(4-AMINOPHENYL)-21H,23H-PORPHINE,5,10,15,20-Tetrakis(4-aminophenyl)-21H,23H-porphyrin
Field of Interest: Metal Organic Frameworks, Nano Particles
Background: meso-Tetra (4-aminophenyl) Porphine is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals. Meso-tetra(4-aminophenyl)porphine is of interest for the construction of metal organic frameworks MOFs, covalent organic frameworks COFs, and nanoparticles. Please contact us if you are looking for a metallated form of this porphyrin that is not listed in our catalog.
References:
1.) Bifunctional covalent organic frameworks with two dimensional organocatalytic micropores, Shinde, Digambar Balaji; Kandambeth, Sharath; Pachfule, Pradip; Kumar, Raya Rahul; Banerjee, Rahul, Chemical Communications (Cambridge, United Kingdom) (2015), 51(2), 310-313.
2.) Manganese porphyrin derivatives as ionophores for thiocyanate-selective electrodes: the influence of porphyrin substituents and additives on the response properties Khorasani, Jafar H.; Amini, Mohammad K.; Motaghi, Hasan; Tangestaninejad, Shahram; Moghadam, Majid, Sensors and Actuators, B: Chemical (2002), 87(3), 448-456.
3.) Positional effects of second-sphere amide pendants on electrochemical CO2 reduction catalyzed by iron porphyrins, Nichols, Eva M.; Derrick, Jeffrey S.; Nistanaki, Sepand K.; Smith, Peter T.; Chang, Christopher J., Chemical Science (2018), 9(11), 2952-2960.
4.) The optical gas-sensing properties of an asymmetrically substituted porphyrin, Pedrosa, Jose Ma; Dooling, Colin M.; Richardson, Tim H.; Hyde, Robert K.; Hunter, Chris A.; Martin, Ma Teresa; Camacho, Luis, Journal of Materials Chemistry (2002), 12(9), 2659-2664.
5.) Micellar Cobaltporphyrin Nanorods in Alcohols, Yuasa, Makoto; Oyaizu, Kenichi; Yamaguchi, Aritomo; Kuwakado, Michi, Journal of the American Chemical Society (2004), 126(36), 11128-11129.
6.) Influence of Molecular Organization of Asymmetrically Substituted Porphyrins on Their Response to NO2 Gas, Pedrosa, Jose M.; Dooling, Colin M.; Richardson, Tim H.; Hyde, Robert K.; Hunter, Chris A.; Martin, Maria T.; Camacho, Luis, Langmuir (2002), 18(20), 7594-7601.
7.) State-of-the-art catechol porphyrin COF catalyst for chemical fixation of carbon dioxide via cyclic carbonates and oxazolidinones, Saptal, Vitthal; Shinde, Digambar Balaji; Banerjee, Rahul; Bhanage, Bhalchandra M., Catalysis Science & Technology (2016), 6(15), 6152-6158.
8.) Spin effects on decay dynamics of charge-separated states generated by photoinduced electron transfer in zinc porphyrin-naphthalenediimide dyads, Mori, Yukie; Sakaguchi, Yoshio; Hayashi, Hisaharu , Journal of Physical Chemistry A (2002), 106(18), 4453-4467.
9.) Stable Binding of Isothiocyanoporphyrin Molecules to Au(111): An STM Study, Han, Wenhai; Li, Shumin; Lindsay, S. M.; Gust, Devens; Moore, Thomas A.; Moore, Ana L., Langmuir (1996), 12(23), 5742-5744.
10.) A porous porphyrin organic polymer (PPOP) for visible light triggered hydrogen production, Mukherjee, Gargi; Thote, Jayshri; Aiyappa, Harshitha Barike; Kandambeth, Sharath; Banerjee, Subhrashis; Vanka, Kumar; Banerjee, Rahul, Chemical Communications (Cambridge, United Kingdom) (2017), 53(32), 4461-4464.
11.) Efficient ternary organic photovoltaics incorporating a graphene-based porphyrin molecule as a universal electron cascade materials, Stylianakis, M. M.; Konios, D.; Kakavelakis, G.; Charalambidis, G.; Stratakis, E.; Coutsolelos, A. G.; Kymakis, E.; Anastasiadis, S. H., Nanoscale (2015), 7(42), 17827-17835.