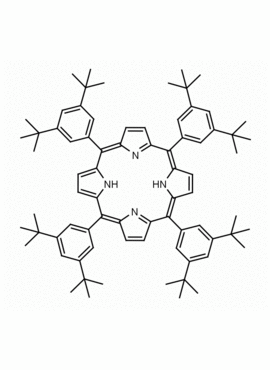
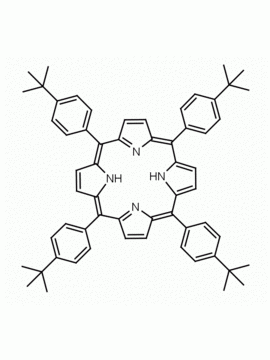
meso-Tetra-(3,5-di-t-butylphenyl)porphine CAS: 89372-90-7 MDL: MFCD01074423
Molecular weight: 1063.59 g/mol
Molecular Formula: C76H94N4
CAS Number: 89372-90-7
Storage: Store at room temperature, protect from light
Synonyms: 2,7,12,17-Tetrakis(
Field of Interest: Synthetic Porphyrins, Dye Sensitized Solar Cells DSSCs,
Background: meso-Tetra-(3,5-di-t-butylphenyl)porphine is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals.
References:
1.) Hampel, et al. Oxidative Cyclodehydrogenation Reactions with Tetraarylporphyrins. European Journal of Organic Chemistry, 2020, vol. 2020, # 43, p. 6758 – 6762. https://doi.org/10.1002/ejoc.202001174
2.) Ladomenou, et al. Ru(II) porphyrins as sensitizers for DSSCs: Axial vs. peripheral carboxylate anchoring group. Journal of Porphyrins and Phthalocyanines, 2019, vol. 23, # 7-8, p. 870 – 880. https://doi.org/10.1142/S108842461950072X
3.) Weber, et al. Cunning metal core: Efficiency/stability dilemma in metallated porphyrin based light-emitting electrochemical cells. Dalton Transactions, 2016, vol. 45, # 34, p. 13284 – 13288. https://doi.org/10.1039/C6DT02293F
4.) Lee, et al. Synthetically tuneable biomimetic artificial photosynthetic reaction centres that closely resemble the natural system in purple bacteria. Chemical Science, 2016, vol. 7, # 10, p. 6534 – 6550. https://doi.org/10.1039/C6SC01076H
5.) Di Carlo, et al. Light-Induced Regiospecific Bromination of meso-Tetra(3,5-di-tert-butylphenyl)Porphyrin on 2,12 β-Pyrrolic Positions. Journal of Organic Chemistry, 2015, vol. 80, # 10, p. 4973 – 4980. https://doi.org/10.1021/acs.joc.5b00367
6.) Jasieniak, et al. Characterization of a porphyrin-containing dye-sensitized solar cell. Journal of Physical Chemistry B, 2004, vol. 108, # 34, p. 12962 – 12971. https://doi.org/10.1021/jp048073i
7.) Di Carlo, et al. Tetraaryl ZnII porphyrinates substituted at β-pyrrolic positions as sensitizers in dye-sensitized solar cells: A comparison with meso-disubstituted push-pull ZnII porphyrinates .Chemistry – A European Journal, 2013, vol. 19, # 32, p. 10723 – 10740. https://doi.org/10.1002/chem.201300219
8.) Flamigni, et al. Switching of electron-to energy-transfer by selective excitation of different chromophores in arrays based on porphyrins and a polypyridyl iridium complex. Journal of Physical Chemistry B, 2002, vol. 106, # 26, p. 6663 – 6671. https://doi.org/10.1021/jp025509q
9.) Eng, et al. Triplet photophysics of gold(III) porphyrins. Journal of Physical Chemistry A, 2005, vol. 109, # 9, p. 1776 – 1784. J. Phys. Chem. A 2005, 109, 9, 1776–1784. https://pubs.acs.org/doi/10.1021/jp0449399
10.) Tamiaki, et al. Induced circular dichroism by complexation of gadolinium(III) porphyrinates with chiral amino acids and dipeptides: Effects of axial β-diketonate ligands on chirality sensing and recognition. Tetrahedron, 2003, vol. 59, # 52, p. 10477 – 10483. https://doi.org/10.1016/j.tet.2003.08.069