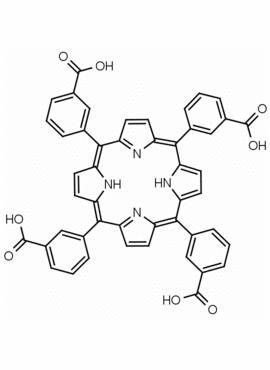
meso-Tetra (3-carboxyphenyl) porphine CAS: 70152-54-4 MDL: MFCD22377424
Molecular weight: 790.77 g/mol
Molecular Formula: C48H30N4O8
CAS Number: 70152-54-4
Storage: Store at room temperature, protect from light.
Synonyms: 3,3′,3”,3”’-(5,10,15,20-Porp
Fields of Interest: Synthetic Porphyrins, Sensors, Molecular Recognition, Supramolecular Chemistry, Metal-Organic Frameworks MOFs, Coordination Polymers
Background: meso-Tetra (3-carboxyphenyl) porphine is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals.
References:
1.) Ueda, et al. Four- and two-armed hetero porphyrin dimers: their specific recognition and self-sorting behaviours. Org. Biomol. Chem., 2022,20, 387-395. https://doi.org/10.1039/D1OB01694F
2.) Roales, et al. Free-Base Carboxyphenyl Porphyrin Films Using a TiO2 Columnar Matrix: Characterization and Application as NO2 Sensors. Sensors 2015, 15(5), 11118-11132; https://doi.org/10.3390/s150511118
3.) Liu, et al. Selectively recognizing organic semiconducting molecules on solid state molecular cages based on ZnOTCPP. Dalton Trans., 2014,43, 432-438. https://doi.org/10.1039/C3DT51609A
4.) Takulapalli, et al. Electrical Detection of Amine Ligation to a Metalloporphyrin via a Hybrid SOI-MOSFET. J. Am. Chem. Soc. 2008, 130, 7, 2226–2233. https://doi.org/10.1021/ja076328a
5.) DaSilva, et al. Novel hybrids of graphitic carbon nitride sensitized with free-base meso-tetrakis(carboxyphenyl) porphyrins for efficient visible light photocatalytic hydrogen production. Applied Catalysis B: Environmental. Volume 221, February 2018, Pages 56-69. https://doi.org/10.1016/j.apcatb.2017.08.079
6.) Lipstman, et al. Supramolecular Crystal Chemistry with Porphyrin Tinkertoys. Hydrogen-Bonding and Coordination Networks with the “Chair” and “Table” Conformers of Tetra(3-carboxyphenyl)porphyrin. Cryst. Growth Des. 2013, 13, 2, 942–952. https://doi.org/10.1021/cg301728r
7.) Lipstman, et al. 2D and 3D coordination networks of tetra(carboxyphenyl)-porphyrins with cerium and thulium ions. Journal of Molecular Structure. Volume 890, Issues 1–3, 12 November 2008, Pages 101-106. https://doi.org/10.1016/j.molstruc.2008.03.044
8.) Muniappan, et al. Porphyrin Framework Solids. Synthesis and Structure of Hybrid Coordination Polymers of Tetra(carboxyphenyl)porphyrins and Lanthanide-Bridging Ions. Inorg. Chem. 2007, 46, 14, 5544–5554. https://doi.org/10.1021/ic0701099