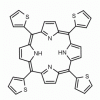
meso-Tetra-(2-thienyl)porphine 5,10,15,20-Tetra-2-thienyl-porphine CAS: 22112-87-4 MDL: MFCD03931638
Molecular weight: 638.85 g/mol
Molecular Formula: C36H22N4S4
CAS Number: 22112-87-4
Storage: Store at room temperature, protect from light
Synonyms: 21H,23H-Porphine, 5,10,15,20-tetra-2-thienyl-, 5,10,15,20-Tetra(2-thienyl)porphyrin, 5,10,15,20-Tetra(2-thienyl)porphyrin, 5,10,15,20-Tétra(2-thiényl)porphyrine, MFCD03931638, 22112-87-4, 2,7,12,17-TETRA(2-THIENYL)-21,22,23,24-TETRAAZAPENTACYCLO[16.2.1.1(3,6).1(8,11).1(13,16)]TETRACOSA-1,3,5,7,9,11(23),12,14,16,18(21),19-UNDECAENE, 2,7,12,17-tetra(2-thienyl)-21,22,23,24-tetraazapentacyclo[16.2.1.13,6.18,11.113,16]tetracosa-1,3,5,7,9,11(23),12,14,16,18(21),19-undecaene, 5,10,15,20-TETRA-2-THIENYL PORPHINE, 5,10,15,20-Tetra-2-thienylporphine, 5,10,15,20-Tetra-2-thienyl-Porphine, H2TTP, tetra(2-thienyl)porphin, tetrathienylporphyrin, tetrathienylporphyrin, H2TTP
Field of Interest: Synthetic Porphyrins,
Background: meso-Tetra-(2-thienyl)porphine is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals.
References:
1.) Okada, et al. Substituent-Control Exciton in J-Aggregates of Protonated Water-Insoluble Porphyrins. J. Am. Chem. Soc. 2003, 125, 9https://doi.org/10.1021/ja017768j
2.) Bhyrappa, et al. Meso-tetrathienylporphyrins: electrochemical and axial ligation properties. Chemical Physics Letters. Volume 349, Issues 5–6, 7 December 2001, Pages 399-404. https://doi.org/10.1016/S0009-2614(01)01189-7
4.) Momo, et al. Chemical Transformations and Photophysical Properties of meso-Tetrathienyl-Substituted Porphyrin Derivatives. EurJOC. Volume 2014, Issue 21, July 2014, Pages 4536-4547. https://doi.org/10.1002/ejoc.201402227
5.) Zhang, et al. New conjugated meso-tetrathienylporphyrin-cored derivatives as two-photon photosensitizers for singlet oxygen generation. Dyes and Pigments, Volume 153, June 2018, Pages 248-255. https://doi.org/10.1016/j.dyepig.2018.01.028
6.) Leite, et al. Remarkable Electronic Effect on the meso-Tetra(thienyl)porphyrins. Inorg. Chem. 2019, 58, 2https://doi.org/10.1021/acs.inorgchem.8b01032