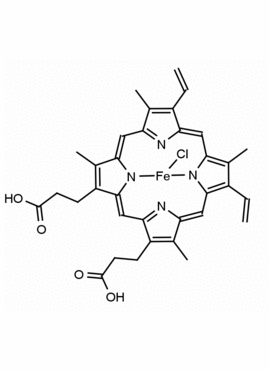
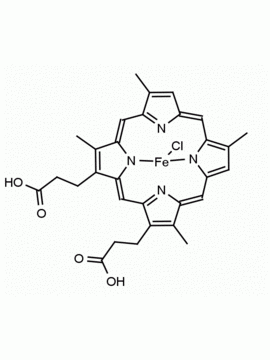
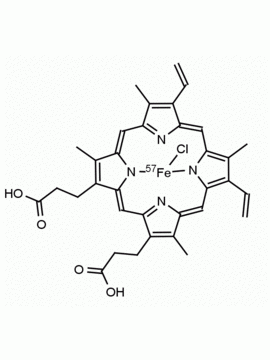
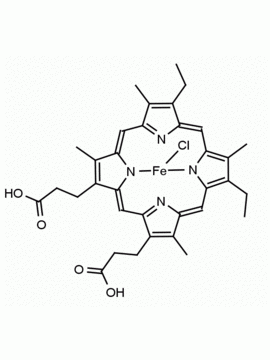
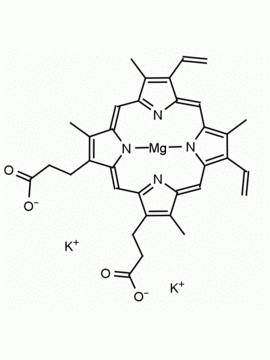
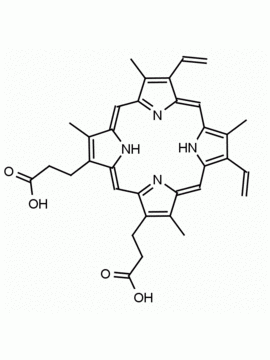
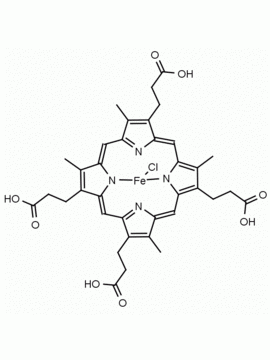
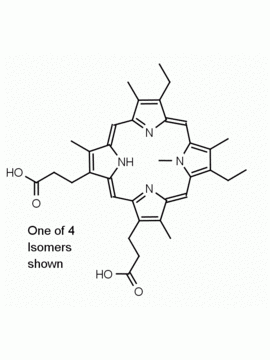
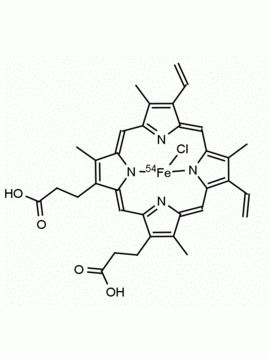
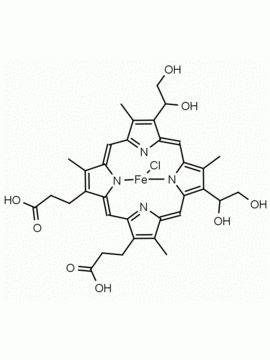
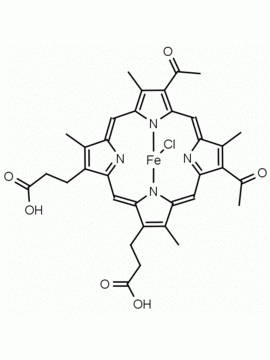
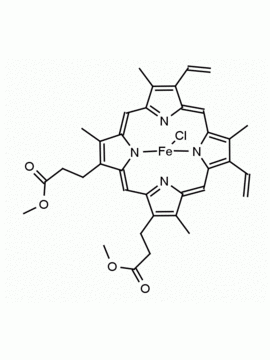
Hemin Ferriprotoporphyrin IX chloride CAS: 16009-13-5 MDL: MFCD00010726
Field of Interest: Natural Products Synthesis, Heme Containing Proteins, Hemoglobin, Myoglobin, Cytochrome C pathology, Complexes, Heme oxygenase
Background: Hemin is the Iron (III) derivative of photoporphyrin IX and natural product which has a myriad of bioactivities including up-regulatation of heme oxygenase 1 (Hmox1, EC 1.14.99.3), which catabolizes heme to biliverdin, carbon monoxide, and free iron, and results in oxidative stress and cellular injury. Aptamer-hemin complexes and other conjugates are found to mimic peroxidases and hemin is used in catalytic nanomaterials.
Molecular weight: 651.95 g/mol
Molecular Formula: C34H32ClFeN4O4
CAS Number: 16009-13-5
Storage: Store at room temperature, protect from light
Synonyms: Ferric Hemin, ferric iron ppIX chloride, ferriheme, Ferriporphyrin Chloride, Ferriprotoporphyrin, Ferriprotoporphyrin ix, Ferriprotoporphyrin IX chloride, haemin
Field of Interest: Natural Products Synthesis, Heme Containing Proteins, Hemoglobin, Myoglobin, Cytochrome C pathology, Complexes, Heme oxygenase
Selected Heme References:
1.) Reduced stress defense in heme oxygenase 1-deficient cells, Poss, Kenneth D., Tonegawa, Susumu, Proceedings of the National Academy of Sciences of the United States of America (1997), 94(20), 10925-10930. DOI:10.1073/pnas.94.20.10925
2.) DNA-enhanced peroxidase activity of a DNA aptamer-hemin complex, Travascio, Paola, Li, Yingfu, Sen, Dipankar, Chemistry & Biology (1998), 5(9), 505-517. DOI:10.1016/S1074-5521(98)90006-0
3.) Chemiluminescent and Chemiluminescence Resonance Energy Transfer (CRET) Detection of DNA, Metal Ions, and Aptamer-Substrate Complexes Using Hemin/G-Quadruplexes and CdSe/ZnS Quantum Dots, Freeman, Ronit, Liu, Xiaoqing, Willner, Itamar, Journal of the American Chemical Society (2011), 133(30), 11597-11604. DOI:10.1021/ja202639m
4.) Therapeutic strategies by modulating oxygen stress in cancer and inflammation, BFang, Jun, Seki, Takahiro, Maeda, Hiroshi, Advanced Drug Delivery Reviews (2009), 61(4), 290-302., DOI:10.1016/j.addr.2009.02.005
5.) Heme oxygenase-1-derived bilirubin ameliorates postischemic myocardial dysfunction, Clark, James E., Foresti, Roberta, Sarathchandra, Padmini, Kaur, Harparkash, Green, Colin J. Motterlini, Roberto, American Journal of Physiology (2000), 278(2, Pt. 2), H643-H651.
6.) A supramolecular-hydrogel-encapsulated hemin as an artificial enzyme to mimic peroxidase, Wang, Qigang, Yang, Zhimou, Zhang, Xieqiu, Xiao, Xudong, Chang, Chi K., Xu, Bing, Angewandte Chemie, International Edition (2007), 46(23), 4285-4289. DOI:10.1002/anie.200700404
7.) Lead(II)-Induced Allosteric G-Quadruplex DNAzyme as a Colorimetric and Chemiluminescence Sensor for Highly Sensitive and Selective Pb2+ Detection, Li, Tao, Wang, Erkang, Dong, Shaojun, Analytical Chemistry (Washington, DC, United States) (2010), 82(4), 1515-1520. DOI:10.1021/ac902638v
8.) DNAzyme-Functionalized Au Nanoparticles for the Amplified Detection of DNA or Telomerase Activity, Niazov, Tamara, Pavlov, Valeri, Xiao, Yi, Gill, Ron, Willner, Itamar, Nano Letters (2004), 4(9), 1683-1687. DOI:10.1021/nl0491428
9.)Hemin: a possible physiological mediator of low density lipoprotein oxidation and endothelial injury, Balla, G., Jacob, H. S., Eaton, J. W., Belcher, J. D., Vercellotti, G. M., Arteriosclerosis and Thrombosis (1991), 11(6), 1700-11., DOI:10.1161/01.ATV.11.6.1700
10.) Colorimetric and fluorescent dual-identification of glutathione based on its inhibition on the 3D ball-flower shaped Cu-hemin-MOF’s peroxidase-like activity, Chen, Xiaolong, Wang, Xianfeng, Cao, Gaihua, Wu, Yawen, Luo, Huibo, Ji, Zhong, Shen, Caihong, Huo, Danqun, Hou, Changjun, Microchimica Acta (2020), 187(11), 601, DOI:10.1007/s00604-020-04565-4
11.) Reactive metamizole metabolites enhance the toxicity of hemin on the ATP pool in HL60 cells by inhibition of glycolysis, Rudin, Deborah, Schmutz, Maurice, Roos, Noemi Johanna, Bouitbir, Jamal, Krahenbuhl, Stephan, Biomedicines (2020), 8(7), 212. DOI:10.3390/biomedicines8070212
12.) Methyl orange removal by a novel PEI-AuNPs-hemin nanocomposite, Hu, Weiwen, Yu, Xuehua, Hu, Qiong, Kong, Jinming, Li, Lianzhi, Zhang, Xueji, Journal of Environmental Sciences (Beijing, China) (2017), 53, 278-283, DOI:10.1016/j.jes.2016.01.01613.
13.) Hemin prevents increased glycolysis in macrophages upon activation: protection by microbiota-derived metabolites of polyphenols, Carrasco-Pozo, Catalina, Tan, Kah Ni, Avery, Vicky M., Antioxidants (2020), 9(11), 1109, DOI:10.3390/antiox9111109
14.) Recent Advances in the Design and Sensing Applications of Hemin/Coordination Polymer-Based Nanocomposites, Alsharabasy, Amir M., Pandit, Abhay, Farras, Pau, Advanced Materials (Weinheim, Germany) (2020), Ahead of Print. DOI:10.1002/adma.202003883
15.) Yachie, Akihiro, Yo, Niida, Wada, Taizo, Igarashi, Noboru, Kaneda, Hisashi, Toma, Tomoko, Ohta, Kazuhide, Kasahara, Yoshihito, Koizumi, Shoichi, Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency, Journal of Clinical Investigation (1999), 103(1), 129-135. DOI:10.1172/JCI4165.
16.) Lin, Youhui, Ren, Jinsong, Qu, Xiaogang, Catalytically active nanomaterials: A promising candidate for artificial enzymes, Accounts of Chemical Research (2014), 47(4), 1097-1105. DOI:10.1021/ar400250z.
17.) Guo, Yujing, Deng, Liu, Li, Jing, Guo, Shaojun, Wang, Erkang, Dong, Shaojun, Hemin-graphene hybrid nanosheets with intrinsic peroxidase-like activity for label-free colorimetric detection of single-nucleotide polymorphism, ACS Nano (2011), 5(2), 1282-1290. DOI:10.1021/nn1029586