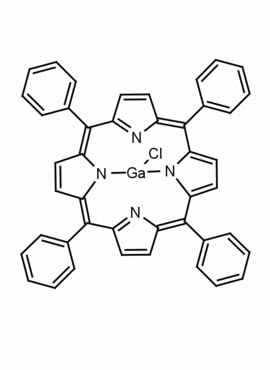
Ga(III) meso-Tetraphenylporphine chloride CAS: 78833-52-0 MDL: MFCD32662380
Molecular weight: 717.90 g/mol
Molecular Formula: C44H28ClGaN4
CAS Number: 78833-52-0
Storage: Store at room temperature, protect from light.
Synonyms: 21H,23H-Porphine, 5
Fields of Interest: Synthetic Porphyrins, Catalysis, Antimicrobial Porphyrins, Synthetic Heme Analogues
Background: Ga(III) meso-Tetraphenylporphine chloride is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals. Ga(III) meso-Tetraphenylporphine chloride display electrocatalytic properties towards the hydrogen evolution reaction.1 Ga(III) meso-Tetraphenylporphine chloride has antimicrobial properties.2 Ga(III) meso-Tetraphenylporphine chloride has been used as a synthetic heme analogue.3,4 The spectroscopic and photophysical properties of Ga(III) meso-Tetraphenylporphine chloride have been reported.5,6
References:
1.) Wang, et al. Electrocatalytic hydrogen evolution with gallium hydride and ligand-centered reduction. Chem. Sci., 2019,10, 2308-2314. https://doi.org/10.1039/C8SC05247F
2.) Choi, et al. In Vitro Efficacy of Free and Nanoparticle Formulations of Gallium(III) meso-Tetraphenylporphyrine against Mycobacterium avium and Mycobacterium abscessus and Gallium Biodistribution in Mice. Mol. Pharmaceutics 2018, 15, 3, 1215–1225. https://doi.org/10.1021/acs.molpharmaceut.7b01036
3.) DiPasquale, et al. Hydrogen Peroxide: A Poor Ligand to Gallium Tetraphenylporphyrin. J. Am. Chem. Soc. 2008, 130, 6, 1812–1813. https://doi.org/10.1021/ja077598w
4.) Meininger, et al. Gallium(III) Tetraphenylporphyrinates Containing Hydrosulfide and Thiolate Ligands: Structural Models for Sulfur-Bound Iron(III) Hemes. Inorg. Chem. 2016, 55, 5, 2421–2426. https://doi.org/10.1021/acs.inorgchem.5b02822
5.) Ebeid, et al. Spectroscopic and stability characteristics of Ga-tetraphenyl porphyrin- and Ge- and Sn-tetra (p-methyl-phenyl) porphyrin chlorides. Spectrochimica Acta Part A: Molecular Spectroscopy. Volume 44, Issue 2, 1988, Pages 127-130. https://doi.org/10.1016/0584-8539(88)80235-6
6.) Ohno, et al. Luminescence of some metalloporphins including the complexes of the IIIb metal group. J. Chem. Phys. 82, 1779 (1985); https://doi.org/10.1063/1.448410