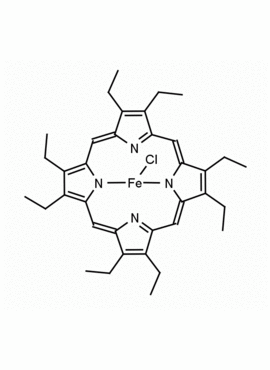
Fe(III) Octaethylporphine chloride CAS: 28755-93-3 MDL: MFCD00011614
Product Name: Fe(III) Octaethylporphine chloride
Catalog Number: O40944
Size: milligram, gram, and larger quantities available.
Molecular Formula: C36H44N4FeCl
MW: 624.059
CAS: 28755-93-3
Storage: Store at room temperature, protect from light
Alternative Names
28755-93-3, Chlor[2,3,7,8,12,13,17,18-octaethylporphyrinato(2-)-κ2N21,N23]eisen,Chloro[2,3,7,8,12,13,17,18-octaethylporphyrinato(2-)-κ2N21,N23]iron,Chloro[2,3,7,8,12,13,17,18-octaéthylporphyrineato(2-)-κ2N21,N23]fer, Iron, chloro[2,3,7,8,12,13,17,18-octaethyl-21H,23H-porphinato(2-)-κN21,κN23], MFCD00011614, 2,3,7,8,12,13,17,18-Octaethyl-21H,23H-porphine iron(III) chloride, 2,3,7,8,12,13,17,18-OCTAETHYL-21H,23H-PORPHINEIRON CHLORIDE, Fe(III) Octaethylporphine chloride, CCc1c(CC)c2cc3c(CC)c(CC)c4cc5nc(cc6c(CC)c(CC)c(cc1n2)n6[Fe](Cl)n34)c(CC)c5CC
Field of Interest: Catalysis, Organic Synthesis, Electrocatalysis
Background: Fe(III) octaethylporphine chloride is a specialty chemical manufactured by Frontier Specialty Chemicals. Fe(III) octaethylporphine chloride supported on glassy carbon acts as an electro catalyst for oxygen reduction.1 The Macmillan reported the use of Fe(III) octaethylporphine chloride as a catalyst for biomimetic sp3 -sp3 cross coupling.2
References:
1.) Khorasani-Motlagh, et al. Iron(III) octaethylporphyrin chloride supported on glassy carbon as an electro catalyst for oxygen reduction. Journal of Electroanalytical Chemistry Volume 565, Issue 1, 1 April 2004, Pages 115-120.
2.) Liu, et al. A biomimetic SH2 cross-coupling mechanism for quaternary sp3-carbon formation. SCIENCE 11 Nov 2021 Vol 374, Issue 6572 pp. 1258-1263 DOI: 10.1126/science.abl43
3.) Abucayon, et al. Lewis Acid Activation of the Ferrous Heme–NO Fragment toward the N–N Coupling Reaction with NO To Generate N2O. J. Am. Chem. Soc. 2018, 140, 12, 4204–4207. https://doi.org/10.1021/jacs.7b13681
4.) Kong, et al. Control of the Chemoselectivity of Metal N-Aryl Nitrene Reactivity: C–H Bond Amination versus Electrocyclization. J. Am. Chem. Soc. 2016, 138, 40, 13271–13280. https://doi.org/10.1021/jacs.6b07026