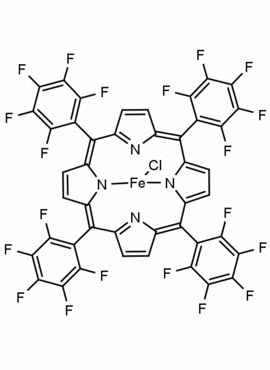
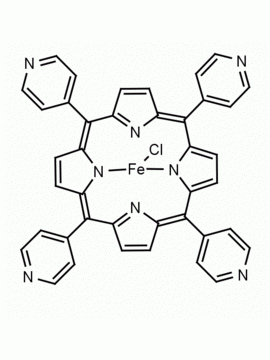
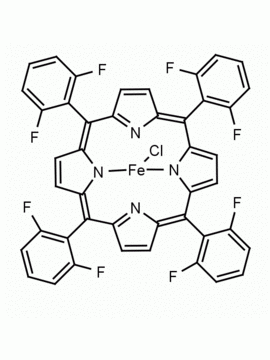
Fe(III) meso-Tetra(Pentafluorophenyl) Porphine Chloride CAS: 36965-71-6 MDL: MFCD00012151
Molecular weight: 1063.827 g/mol
Molecular Formula: C44H8ClF20FeN4
CAS Number: 36965-71-6
Storage: Store at room temperature, protect from light.
Synonyms: 36965-71-6, Chlor[5,10,15,20-te
Fields of Interest: Catalysis, Cytochrome P450 mimics, Electrophilic Halogenation, Biomimetic Oxidation
Background: Fe(III) meso-Tetra(Pentafluorophenyl) Porphine Chloride is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals. Fe(III) meso-Tetra(Pentafluorophenyl) Porphine Chloride is commonly used for biomimetic oxidations.1-5
References:
1.) Balogh-Weiser, et al. Magnetic Nanoparticles with Dual Surface Functions—Efficient Carriers for Metalloporphyrin-Catalyzed Drug Metabolite Synthesis in Batch and Continuous-Flow Reactors. Nanomaterials 2020, 10, 2329. https://doi.org/10.3390/nano10122329
2.) Engbers, et al. Toward Environmentally Benign Electrophilic Chlorinations: From Chloroperoxidase to Bioinspired Isoporphyrins, Inorg. Chem. 2022, 61, 21, 8105–8111. https://doi.org/10.1021/acs.inorgchem.2c00602
3.) Hiroshi, et al. Characterization of High Valent Iron Porphyrin in Catalytic Reaction by Iron(III) Tetrapentafluorophenylporphyrin. Chemistry Letters, 1994, Vol.23, No.8, 1491-1494. https://www.journal.csj.jp/doi/abs/10.1246/cl.1994.1491
4.) Franke, et al. Axial Ligand and Spin-State Influence on the Formation and Reactivity of Hydroperoxo–Iron(III) Porphyrin Complexes. Chemistry, a European Journal.Volume 18, Issue 22, May 29, 2012, Pages 6935-6949. https://doi.org/10.1002/chem.201103036
5.) Pereira, et al. Metalloporphyrins: Bioinspired Oxidation Catalysts. ACS Catal. 2018, 8, 11, 10784–10808. https://doi.org/10.1021/acscatal.8b01871