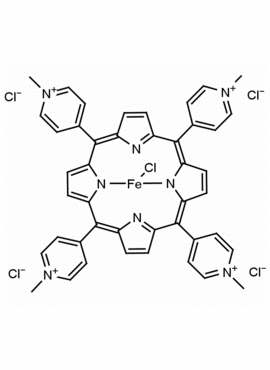
Fe(III) meso-Tetra (N-Methyl-4-Pyridyl) porphine pentachloride CAS: 133314-07-5 MDL: MFCD01866633
Molecular weight: 909.9 g/mol
Molecular Formula: C44H36FeN8
CAS Number: 133314-07-5
Storage: Store at room temperature, protect from light.
Purity: >95%
Synonyms: FeTMPyP pentachloride, 133314-07-5, CTK8E8442, DTXSID90849546, Iron(III) meso -tetra(N-methyl-4-pyridyl)porphine pentachloride, Chlor{4,4′,4”,4”’
Uses: catalysis, reactive oxygen species inhibition, electrocatalysis
Background: Fe(III) meso-Tetra (N-Methyl-4-Pyridyl) porphine pentachloride is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals. Fe(III) meso-Tetra (N-Methyl-4-Pyridyl) porphine pentachloride acts as a peroxynitrite decomposition catalyst in-vivo.1 Fe(III) meso-Tetra (N-Methyl-4-Pyridyl) porphine pentachloride inhibits reactive oxygen species in a rat model of ischemia repercussion injury.2 Fe(III) meso-Tetra (N-Methyl-4-Pyridyl) porphine pentachloride acts an electrocatalyst towards the reduction of NO.3 Fe(III) meso-Tetra (N-Methyl-4-Pyridyl) porphine pentachloride in combination with H3PMo12O40 self assembles onto MWCNTs and acts as an electrocatalyst for the oxygen reduction reaction.4 Fe(III) meso-Tetra (N-Methyl-4-Pyridyl) porphine pentachloride was used to dope carbon nano sheets for the synthesis of a CO2 Electroreduction catalyst.5
References:
1.) Peroxynitrite decomposition catalysts: therapeutics for peroxynitrite-mediated pathology, Salvemini, Daniela; Wang, Zhi-Qiang; Stern, Michael K.; Currie, Mark G.; Misko, Thomas P., Proceedings of the National Academy of Sciences of the United States of America (1998), 95(5), 2659-2663 DOI:10.1073/pnas.95.5.2659
2.) Inhibition of reactive oxygen species downregulates the MAPK pathway in rat spinal cord after limb ischemia reperfusion injury, Choi Eun Kyung; Yeo Jin-Seok; Park Chan Yoon; Na Ho in; Lim Jung a; Lee Jeong-Eun; Hong Seong Wook; Park Sung-Sik; Lim Dong Gun; Kwak Kyung Hwa, International journal of surgery (London, England) (2015), 2274-8.
3.) Chen, et al. Redox Mechanism of NO in Water-Soluble Iron Porphyrin. Electroanalysis. Volume 13, Issue 13, September 2001, Pages 1076-1081. https://doi.org/10.1002/1521-4109(200109)13:13<1076::AID-ELAN1076>3.0.CO;2-Y
4.) Han, et al. Ionic self-assembly of metalloporphyrin/heteropolyacid on multi-wall carbon nanotubes with enhanced electrocatalytic activity toward oxygen reduction reaction. Journal of Porphyrins and Phthalocyanines Vol. 23, No. 03, pp. 235-242 (2019). https://doi.org/10.1142/S1088424619500123
5.) Tuo, et al. Layered Confinement Reaction: Atomic-level Dispersed Iron–Nitrogen Co-Doped Ultrathin Carbon Nanosheets for CO2 Electroreduction. ChemSusChem. Volume 12, Issue 12, June 21, 2019, Pages 2644-2650. https://doi.org/10.1002/cssc.201901058