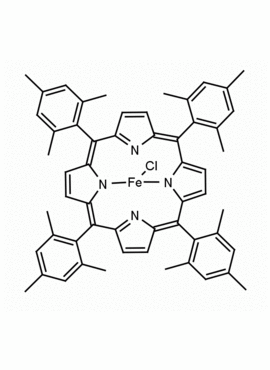
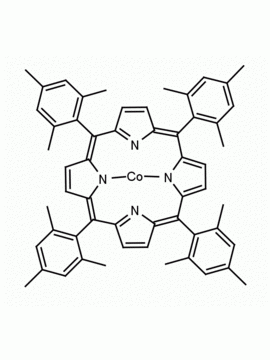
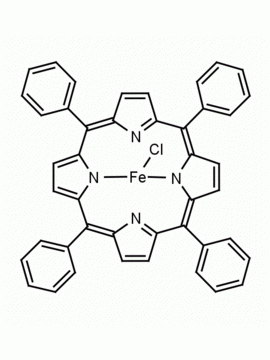
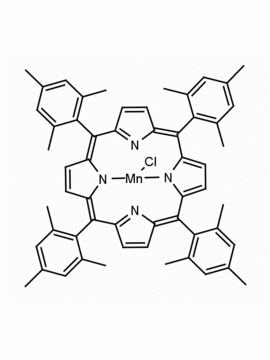
Fe(III) meso-Tetra (2,4,6 trimethylphenyl) Porphine Chloride CAS: 77439-21-5 MDL: MFCD32267975
Molecular weight: 872.34 g/mol
Molecular Formula: C56H52ClFeN4
CAS Number: 77439-21-5
Storage: Store at room temperature, protect from light.
Synonyms: 5,10,15,20-tetra(2′,4′,6′-trimethylphenyl)porphyrinatoiron(III) chloride, FeIII(TMP)Cl, Fe(III) meso-Tetra (2,4,6 trimethylphenyl) Porphine Chloride, 77439-21-5, MFCD32267975
Fields of Interest: Synthetic Porphyrins, Iron (III) Porphyrins, Catalysis, Epoxidation , Cytochrome P450 Mimics, Biomimetic Oxidation
Background: Fe(III) meso-Tetra (2,4,6 trimethylphenyl) Porphine Chloride is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals. High valent iron porphyrin intermediates generated from Fe(III) meso-Tetra (2,4,6 trimethylphenyl) Porphine Chloride are powerful epoxidation catalysts and mimics of cytochrome P450.1-4 Fe(III) meso-Tetra (2,4,6 trimethylphenyl) Porphine Chloride is a catalyst for the stereoselective cyclopropanation of alkenes.3 Several recent mechanistic and fundamental studies have recently been published on this compound.5-6
References:
1.) Groves, et al. High-valent iron-porphyrin complexes related to peroxidase and cytochrome P-450. J. Am. Chem. Soc. 1981, 103, 10, 2884–2886. https://doi.org/10.1021/ja00400a075
2.) Groves, et al. Epoxidation reactions catalyzed by iron porphyrins. Oxygen transfer from iodosylbenzene. J. Am. Chem. Soc. 1983, 105, 18, 5786–5791. https://doi.org/10.1021/ja00356a015
3.) Wolf, et al. Shape and stereoselective cyclopropanation of alkenes catalyzed by iron porphyrins. J. Am. Chem. Soc. 1995, 117, 36, 9194–9199. https://doi.org/10.1021/ja00141a011
4.) Melo, et al. Biomimetic oxidation of praziquantel catalysed by metalloporphyrins. Journal of Molecular Catalysis A: Chemical.Volume 226, Issue 1, 1 February 2005, Pages 23-31. https://doi.org/10.1016/j.molcata.2004.09.015
5.) Chen, et al. Ligand control in the photochemical generation of high-valent porphyrin-iron–oxo derivatives. Chem. Commun., 2015,51, 9949-9952. https://doi.org/10.1039/C5CC02852C
6.) Nishikawa, et al. Spectroscopic Evidence for Acid-Catalyzed Disproportionation Reaction of Oxoiron(IV) Porphyrin to Oxoiron(IV) Porphyrin π-Cation Radical and Iron(III) Porphyrin. J. Am. Chem. Soc. 2020, 142, 11, 4980–4984. https://doi.org/10.1021/jacs.9b13503