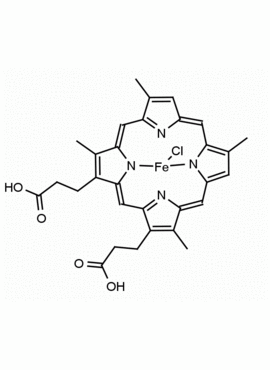
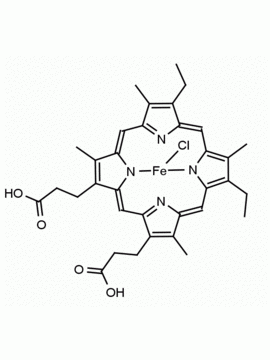
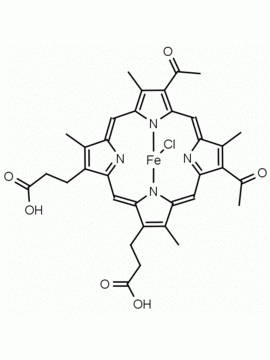
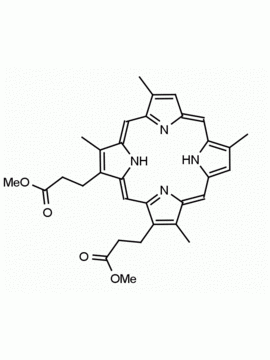
Fe(III) Deuteroporphyrin IX chloride Deutero-hemin CAS: 21007-21-6 MDL: MFCD18070953
Molecular weight: 599.866 g/mol
Molecular Formula: C30H26ClFeN4O4
CAS Number: 21007-21-6
Storage: Store at room temperature, protect from light
Synonyms: Eisen(3+)hydrogench
Field of Interest: Chemistry of Heme derivatives, Coordination Chemistry, Malaria Pigment, Oxidation Chemistry, Cytochrome P450 models
Background: Fe(III) Deuteroporphyrin IX chloride is a natural porphyrin specialty chemical manufactured by Frontier Specialty Chemicals. Fe(III) Deuteroporphyrin IX chloride is useful for studying the chemistry of heme due to its higher stability, compared to heme.
References:
1.) Wyllie, et al. NO Orientation and Tilting in (Nitrosyl)iron(II) Deuteroporphyrin IX. Inorg. Chem. 2003, 42, 14, 4259–4261. https://doi.org/10.1021/ic034364e
2.) Kelly, et al. Chlorite ion oxidation of the iron(III) complex of deuteroporphyrin IX. Inorg. Chem. 1981, 20, 4, 1086–1090. https://doi.org/10.1021/ic50218a026
3.) Teng, et al. Singlet Oxygen Generation in Ferriporphyrin-Polymer Dots Catalyzed Chemiluminescence System for Cancer Therapy. ACS Appl. Bio Mater. 2020, 3, 8, 5020–5029. https://doi.org/10.1021/acsabm.0c00522
4.) Kuter, et al. Hydrating the Bispropionate Notch in Malaria Pigment: A New Structural Motif in the Iron(III)(deuteroporphyrin) Dimer. Chemistry, a European Journal. Volume 25, Issue 17, March 21, 2019, Pages 4373-4378. https://doi.org/10.1002/chem.201805116
5.) Zhou, et al. Metallo-deuteroporphyrin as a novel catalyst for p-xylene oxidation using molecular oxygen. Inorganica Chimica Acta. Volume 382, 15 March 2012, Pages 167-170.
6.) Brault, et al. Reactions of iron(III) porphyrins with peroxyl radicals derived from halothane and halomethanes. J. Phys. Chem. 1984, 88, 13, 2857–2862. https://doi.org/10.1021/j150657a038