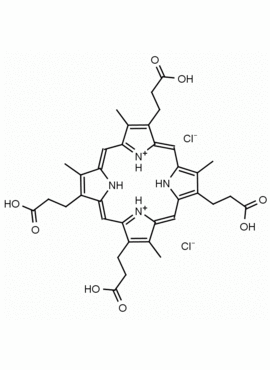
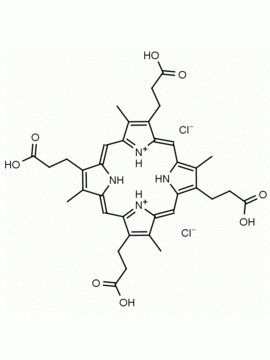
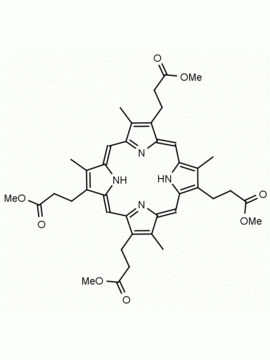
Coproporphyrin III dihydrochloride 3,8,13,17-Tetramethyl-21H,23H-porphine-2,7,12,18-tetrapropionic acid dihydrochloride CAS: 14643-66-4 MDL: MFCD00058927
Field of Interest: Heme Metabolism, Rare Diseases, Porphyrias, Mitochondria
Background: Coproporphyrin a porphyrin based metabolic product of heme catabolism implicated in porphyria diseases and in heavy metal exposure1,2 This specialty chemical has photosensitizing properties and has shown to form ordered micelles and aggregates under aqueous conditions.3,4 Copropoprhyrin I and III can be analyzed by HPLC and LCMS.5,6
Molecular weight: 727.63 g/mol
Molecular Formula: C36H38N4O8 • 2HCl
CAS Number: 14643-66-4
Storage: Store at room temperature, protect from light
Synonyms: Coproporphyrin III, 14643-66-4, Zincphyrin, 21H,23H-Porphine-2,7,12,18-tetrapropanoic acid, 3,8,13,17-tetramethyl-, Zinc coproporphyrin III, 3,8,13,17-tetramethylporphyrin-2,7,12,18-tetrapropanoic acid, Koproporphyrin, Koproporphyrin III
References:
1.) B Cribier, D Rey, G Uhl, C Le Coz, C Hirth, E Libbrecht, D Vetter, J M Lang, F Stoll-Keller, Abnormal urinary coproporphyrin levels in patients infected by hepatitis C virus with or without human immunodeficiency virus. A study of 177 patients, Arch Dermatol, 1996 Dec;132(12):1448-52.
2.) Mahaffey, Kathryn R.; Capar, Stephen G.; Gladen, Beth C.; Fowler, Bruce A., Concurrent exposure to lead, cadmium, and arsenic. Effects on toxicity and tissue metal concentrations in the rat, Journal of Laboratory and Clinical Medicine (1981), 98(4), 463-81.
3.) Finlay, Jarod C.; Conover, David L.; Hull, Edward L.; Foster, Thomas H., Porphyrin bleaching and PDT-induced spectral changes are irradiance dependent in ALA-sensitized normal rat skin in vivo, Photochemistry and Photobiology (2001), 73(1), 54-63.
4.) Brown, Stanley B.; Shillcock, Margaret; Jones, Peter, Equilibrium and kinetic studies of the aggregation of porphyrins in aqueous solution, Biochemical Journal (1976), 153(2), 279-85. Language: English, Database: CAPLUS
5.) High-performance liquid chromatography of uroporphyrin and coproporphyrin isomers. Lim ,et al. Methods in Enzymology Volume 123, 1986, Pages 383-389. https://doi.org/10.1016/S0076-6879(86)23046-3
6.) A fully automated and validated human plasma LC–MS/MS assay for endogenous OATP biomarkers coproporphyrin-I and coproporphyrin-III. King-Ahmad, et al. BIOANALYSIS VOL. 10, NO. 9. https://doi.org/10.4155/bio-2017-0270