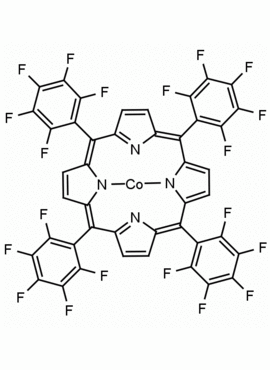
Co(II) meso-Tetra (Pentafluorophenyl) porphine CAS: 52242-06-5 MDL: MFCD09038961
Molecular weight: 1031.462 g/mol
Molecular Formula: C44H8CoF20N4
CAS Number: 52242-06-5
Storage: Store at room temperature, protect from light
Synonyms: 21H,22H-Porphine, 5
Field of Interest: Catalysis, Fluorinated Porphyrins, Synthetic Porphyrins,
Background: Co(II) meso-Tetraphenylporphine (contains 1-3% chlorin) is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals.
References:
1.) Behar, et al. Cobalt Porphyrin Catalyzed Reduction of CO2. Radiation Chemical, Photochemical, and Electrochemical Studies. J. Phys. Chem. A 1998, 102, 17, 2870–2877. https://doi.org/10.1021/jp9807017
2.) Liu, et al. Single-Walled Carbon Nanotube/Metalloporphyrin Composites for the Chemiresistive Detection of Amines and Meat Spoilage. Angew. Chemie. Int. Ed. Volume 54, Issue 22, May 26, 2015, Pages 6554-6557. https://doi.org/10.1002/anie.201501434
3.) Sun, et al. Slow Electron Transfer Rates for Fluorinated Cobalt Porphyrins: Electronic and Conformational Factors Modulating Metalloporphyrin ET. Inorg. Chem. 2003, 42, 19, 6032–6040. https://doi.org/10.1021/ic034705o
4.) Zhang, et al. Co-Catalyzed Transannulation of Pyridotriazoles with Isothiocyanates and Xanthate Esters. Org. Lett. 2020, 22, 21, 8500–8504. https://doi.org/10.1021/acs.orglett.0c03099
5.) Lei, et al. First-row transition metal porphyrins for electrocatalytic hydrogen evolution — a SPP/JPP Young Investigator Award paper. Journal of Porphyrins and Phthalocyanines. Vol. 24, No. 11n12, pp. 1361-1371 (2020). https://doi.org/10.1142/S1088424620500157
6.) Lei, et al. Significantly boosted oxygen electrocatalysis with cooperation between cobalt and iron porphyrins. Dalton Trans., 2021,50, 5120-5123. https://doi.org/10.1039/D1DT00441G