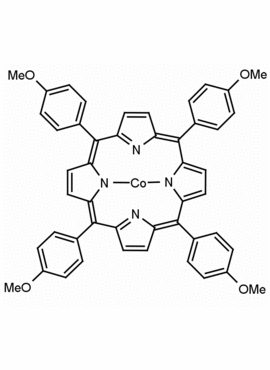
Co(II) meso-Tetra (4-methoxyphenyl) Porphine (1-3% chlorin) Arsenite Ionophore I CAS: 28903-71-1 MDL: MFCD00010724
Molecular weight: 791.76 g/mol
Molecular Formula:C48H35CoN4O4
CAS Number: 28903-71-1
Storage: Store at room temperature, protect from light.
Purity: >95%
Synonyms: Co(II) meso-Tetra (4-methoxyphenyl) Porphine (1-3% chlorin) Arsenite Ionophore I CAS: 28903-71-1 MDL: MFCD00010724
Field of Interest: Catalysis, organic synthesis, electrocatalysis, sensors, ion selective electrodes
Background: Co(II) meso-Tetra (4-methoxyphenyl) porphine (1-3% chlorin) has been used as a selective potentiometric sensor for arsenite and molybdate in aqueous media1,2. Electrochemical oxygen reduction reaction activity has been observed with this porphyrin3,4. C-O Bond cleavage of alcohols via visible light activation of cobalt alkoxycarbonyls derived from Co(II) meso-tetra(4-Methoxyphenyl)porphine5. Co(II) porphyrins are highly efficient catalysts towards desulfurization of dibenzothiophene in fuel oils6. T(p-OMe)PPCo is an effective catalyst for the aerobic oxidative coupling of phenols7. Cobalt(II) porphyrins are effective catalysts for selective cyclopropanation of alkenes with ethyl diazoacetate8.
References:
1.) Gupta, V.K., Agarwal, S.; PVC based 5,10,15,20-tetrakis (4-methoxyphenyl)porphyrinatocobalt(II) membrane potentiometric sensor for arsenite, Talanta65, 2005, 730-734. https://doi.org/10.1016/j.talanta.2004.07.043
2.) Gupta, V.K., Chandra, S., Chauhan, D.K., Mangla, R.; Membranes of 5,10,15,20-Tetrakis(4-Methoxyphenyl) Porphyrinatocobalt(TMOPP-Co) (I) as MoO42-– Selective Sensors. Sensors 2, 2002, 164-173. https://doi.org/10.3390/s20500164
3.) Zhou, Y., Xing, Y.F., Wen, J., Ma, H.B., Wang, F.B., Xia, X.H.; Axial ligands tailoring the ORR activity of cobalt porphyrin. Science Bulletin64, 2019, 1158-1166. https://doi.org/10.1016/j.scib.2019.07.003
4.) Wang, Y.H., Schneider, P.E., Goldsmith, Z.K., Mondal, B., Hammes-Schiffer, S., Stahl, S.S. Bronsted Acid Scaling Relationships Enable Control Over Product Selectivity from O2Reduction with a Mononuclear Cobalt Porphyrin Catalyst. ACS Cent. Sci. 2019, 5, 6, 1024-1034. https://doi.org/10.1021/acscentsci.9b00194
5.) Chambers, D.R., Juneau, A., Ludwig, C.T., Frenette, M., Martin, D.B.C.; C-O Bond Cleavage of Alcohols via Visible Light Activation of Cobalt Alkoxycarbonyls. Organometallics2019, 38, 24, 4570-4577. https://doi.org/10.1021/acs.organomet.9b00552
6.) Tripathi, D., Yadav, I., Negi, H., Singh, R.K., Srivastava, V.C., Sankar, M.; Highly efficient Co(II) porphyrin catalysts for the extractive oxidative desulfurization of dibenzothiophene in fuel oils under mild conditions. Journal of porphyrins and Phthalocyanines 2021, 25, 1, 24-30. https://doi.org/10.1142/S1088424620500443
7.) Jiang, Q., Sheng, W., Tian, M., Tang, J., Guo, C.; Cobalt(II)-Porphyrin-Catalyzed Aerobic Oxidation: Oxidative Coupling of Phenols. Joc. 2013, 10, 1861-1866. https://doi.org/10.1002/ejoc.201201595
8.) Huang, L., Chen, Y., Gao, G.Y., Zhang, X.P.; Diastereoselective and Enantioselective Cyclopropanation of Alkenes Catalyzed by Cobalt Porphyrins. Org. Chem. 2003, 68, 21, 8179-8184. https://doi.org/10.1021/jo035088o