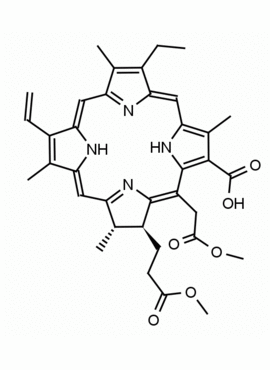
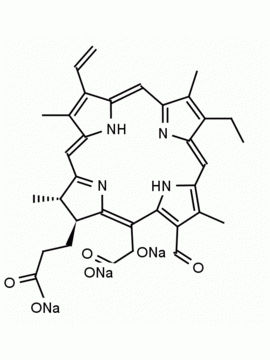
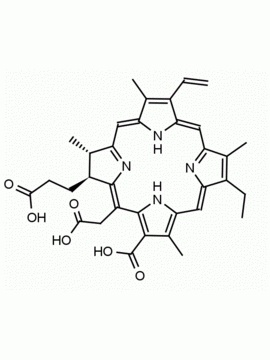
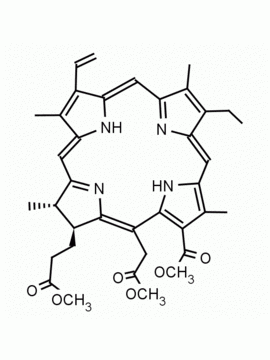
Chlorin e6 dimethylester CAS: 197444-89-6 MDL: MFCD32267982
Molecular weight: 624.73 g/mol
Molecular Formula: C36H40N4O6
CAS Number: 197444-89-6
Storage: Store at room temperature, protect from light.
Synonyms: (17S,18S)-7-Ethyl-2
Field of Interest: Natural porphyrins, Chlorophyll Derivatives
Background: Chlorin e6 dimethylester is a chlorophyll derived natural chlorin specialty chemical derivative manufactured by Frontier Specialty Chemicals. Chlorin e6 dimethylester is used a starting material for the synthesis of chlorin based photosensitizers.
References:
1.) Hargus, et al. Mono-(L)-aspartylchlorin-e6 Photochemistry and Photobiology, 2007, vol. 83, # 5, p. 1006 – 1015. https://doi.org/10.1111/j.1751-1097.2007.00092.x
2.) Jinadasa, et al. Syntheses and cellular investigations of 173-, 152-, and 131-amino acid derivatives of chlorin e6. Journal of Medicinal Chemistry, 2011, vol. 54, # 21, p. 7464 – 7476. https://doi.org/10.1021/jm2005139
3.) Jalde, et al. Synthesis of novel Chlorin e6-curcumin conjugates as photosensitizers for photodynamic therapy against pancreatic carcinoma. European Journal of Medicinal Chemistry, 2018, vol. 147, p. 66 – 76. https://doi.org/10.1016/j.ejmech.2018.01.099
4.) Chen, et al. Syntheses and PDT activity of new mono- and di-conjugated derivatives of chlorin e6 . Journal of Porphyrins and Phthalocyanines, 2017, vol. 21, # 4-6, p. 354 – 363. https://doi.org/10.1142/S1088424617500262
5.) Zhang, et al. Design, synthesis and biological evaluation of novel 31-hexyloxy chlorin e6-based 152– or 131-amino acid derivatives as potent photosensitizers for photodynamic therapy. European Journal of Medicinal Chemistry, 2020, vol. 207, art. no. 112715. https://www.sciencedirect.com/science/article/abs/pii/S0223523420306875?via%3Dihub
6.) Gomi, et al. The Structures of Mono-L-aspartyl chlorin e6 and its Related Compounds. Heterocycles, 1998, vol. 48, # 11, p. 2231 – 2244. DOI: 10.3987/COM-98-8286