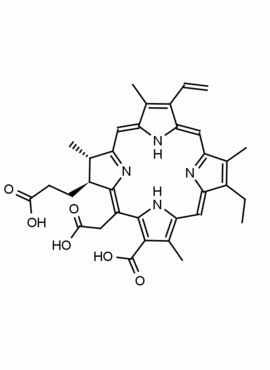
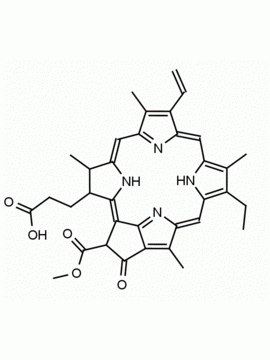
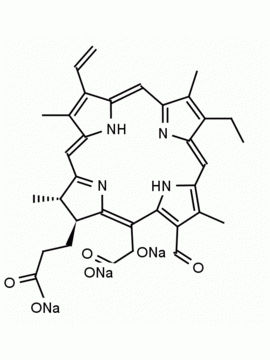
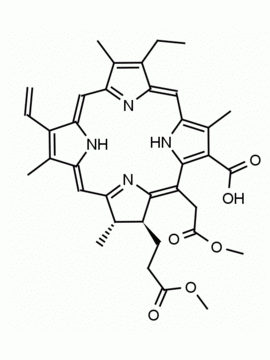
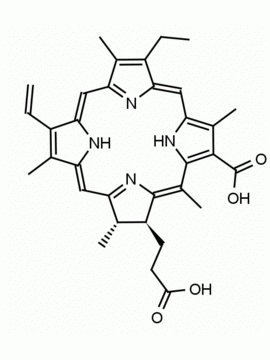
Chlorin e6 CAS: 19660-77-6 MDL: MFCD08669566
Molecular Weight: 596.98
Molecular Formula: C34H36N4O6
CAS Number: 19660-77-6
MFCD: 08669566
Storage: Store at -20 °C, protect from light.
Purity: >95%
Alternative Names
(17S,18S)-18-(2-carboxyethyl)-20-(carboxymethyl)-12-ethenyl-7-ethyl-3,8,13,17-tetramethyl-17,18-dihydroporphyrin-2-carboxylic acid; (17S,18S)-18-(2-Carboxyethyl)-20-(carboxymethyl)-7-ethyl-3,8,13,17-tetramethyl-12-vinyl-17,18-dihydro-2-porphyrincarbonsäure; (17S,18S)-18-(2-Carboxyethyl)-20-(carboxymethyl)-7-ethyl-3,8,13,17-tetramethyl-12-vinyl-17,18-dihydro-2-porphyrincarboxylic acid; (17S,18S)-18-(2-Carboxyethyl)-20-(carboxymethyl)-7-ethyl-3,8,13,17-tetramethyl-12-vinyl-17,18-dihydroporphyrin-2-carboxylic acid; 21H,22H-Porphine-13-propanoic acid, 17-carboxy-15-(carboxymethyl)-7-ethenyl-2-ethyl-12,13-dihydro-3,8,12,18-tetramethyl-, (12S,13S)-; Acide (17S,18S)-18-(2-carboxyéthyl)-20-(carboxyméthyl)-7-éthyl-3,8,13,17-tétraméthyl-12-vinyl-17,18-dihydro-2-porphyrinecarboxylique; Chlorine a6; Chlorine e6; Phytochlorin e6; Phytochlorine e; (17S,18S)-18-(2-carboxyethyl)-20-(carboxymethyl)-12-ethenyl-7-ethyl-3,8,13,17-tetramethyl-17,18,22,23-tetrahydroporphyrin-2-carboxylic acid; (2S-trans)-18-carboxy-20-(carboxymethyl)-13-ethyl-2,3-dihydro-3,7,12,17-tetramethyl-8-vinyl-21H,23H-porphine-2-propionic acid; 3(S),7,12,17-Tetramethyl-13-ethyl-8-ethenyl-2,3-dihydro-18-carboxy-20-(carboxymethyl)-21H,23H-porphine-2(S)-propanoic acid; methyl (2S-trans)-13-ethyl-2,3-dihydro-18-(methoxycarbonyl)-20-(2-methoxy-2-oxoethyl)-3,7,12,17-tetramethyl-8-vinyl-21H,23H-porphine-2-propionate; Phytochlorin
Field of Interest: Chlorophyll Derivatives, Photosensitizers, Chlorins
Background: Frontier Specialty Chemicals provides high purity chlorin e6 for your research and development applications. Please contact us if you need a quantity that is larger than what is listed in our online catalog. Chlorin e6 and derivatives have been extensively investigated for their unique photosensitization properties with applications in chemotherapy of cancers, imaging agents, photodynamic antimicrobial agents and photosensitizers for solar cells and catalysis. Frontier Specialty Chemicals has over 40 years of experience with the unique challenges of chlorin e6 chemistry, including the synthesis of functionalized derivatives for a myriad of applications.
References:
1.) Tegos, et al. Protease-stable polycationic photosensitizer conjugates between polyethyleneimine and chlorin(e6) for broad-spectrum antimicrobial photoinactivation. Antimicrobial Agents and Chemotherapy, 2006, vol. 50, # 4, p. 1402 – 1410. https://doi.org/10.1128/AAC.50.4.1402-1410.2006
2.) Luo, et al. Tumor-targeted hybrid protein oxygen carrier to simultaneously enhance hypoxia-dampened chemotherapy and photodynamic therapy at a single dose. Theranostics, 2018, vol. 8, # 13, p. 3584 – 3596. doi:10.7150/thno.25409
3.) Zhang, et al. Lipid micelles packaged with semiconducting polymer dots as simultaneous MRI/photoacoustic imaging and photodynamic/photothermal dual-modal therapeutic agents for liver cancer. Journal of Materials Chemistry B, 2016, vol. 4, # 4, p. 589 – 599. https://doi.org/10.1039/C5TB01827G
4.) Jinadasa, et al. Syntheses and cellular investigations of 173-, 152-, and 131-amino acid derivatives of chlorin e6. Journal of Medicinal Chemistry, 2011, vol. 54, # 21, p. 7464 – 7476. https://doi.org/10.1021/jm2005139
5.) Sreenilayam, et al. Stereoselective Olefin Cyclopropanation under Aerobic Conditions with an Artificial Enzyme Incorporating an Iron-Chlorin e6 Cofactor. ACS Catalysis, 2017, vol. 7, # 11, p. 7629 – 7633. https://doi.org/10.1021/acscatal.7b02583
6.) Wang, et al. Natural chlorophyll-related porphyrins and chlorins for dye-sensitized solar cells. Molecules, 2012, vol. 17, # 4, p. 4484 – 4497. https://doi.org/10.3390/molecules17044484
7.) Paul, et al. Optimization in solvent selection for chlorin e6 in photodynamic therapy. Journal of Fluorescence, 2013, vol. 23, # 2, p. 283 – 291. https://doi.org/10.1007/s10895-012-1146-x
8.) Stallivieri, et al. Folic acid conjugates with photosensitizers for cancer targeting in photodynamic therapy: Synthesis and photophysical properties. Bioorganic and Medicinal Chemistry, 2017, vol. 25, # 1, p. 1 – 10. https://doi.org/10.1016/j.bmc.2016.10.004
9.) John, et al. Dual Stimuli-Responsive Vesicular Nanospheres Fabricated by Lipopolymer Hybrids for Tumor-Targeted Photodynamic Therapy. Biomacromolecules, 2016, vol. 17, # 1, p. 20 – 31. https://doi.org/10.1021/acs.biomac.5b01474
10.) Paul, et al. Elucidation of monomerization effect of PVP on chlorin e6 aggregates by spectroscopic, chemometric, thermodynamic and molecular simulation studies. Journal of Fluorescence, 2013, vol. 23, # 5, p. 1065 – 1076. https://doi.org/10.1007/s10895-013-1236-4
11.) Tarrago-Trani, et al. Shiga-like toxin subunit B (SLTB)-enhanced delivery of chlorin e6 (Ce6) improves cell killing. Photochemistry and Photobiology, 2006, vol. 82, # 2, p. 527 – 537. https://doi.org/10.1562/2005-06-20-RA-583
12.) Yan, et al. Firmly anchored photosensitizer Chlorin e6 to layered double hydroxide nanoflakes for highly efficient photodynamic therapy in vivo. Chemical Communications, 2017, vol. 53, # 15, p. 2339 – 2342. https://doi.org/10.1039/C6CC09510K
13.) Gladkova, et al. FTIR spectra and normal-mode analysis of chlorin e6 and its degradation-induced impurities. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2010, vol. 76, # 3-4. p. 388 – 394. https://doi.org/10.1016/j.saa.2010.03.037
14.) Zhang, et al. Photodynamic micelles for amyloid β degradation and aggregation inhibition. Chemical Communications, 2016, vol. 52, # 81, p. 12044 – 12047. https://doi.org/10.1039/C6CC06175C
15.) Fan, et al. An MTH1-targeted nanosystem for enhanced PDT: Via improving cellular sensitivity to reactive oxygen species. Chemical Communications, 2018, vol. 54, # 34, p. 4310 – 4313. https://doi.org/10.1039/C8CC01841C
16.) Radestocks, et al. Induction of apoptosis in HaCaT cells by photodynamic therapy with chlorin e6 or pheophorbide a. Skin Pharmacology and Physiology, 2007, vol. 20, # 1, p. 3 – 9. https://doi.org/10.1159/000096166
17.) Suvorov, et al. Synthesis of PSMA-targeted 131– and 152-substituted chlorin e6 derivatives and their biological properties. Journal of Porphyrins and Phthalocyanines, 2018, vol. 22, # 11, p. 1030 – 1038. https://doi.org/10.1142/S1088424618501006
18.) Bi, et al. Potent inhibition of Severe Acute Respiratory Syndrome Coronavirus 2 by photosensitizers compounds. Dyes and Pigments, 2021, vol. 194. 109570. https://doi.org/10.1016/j.dyepig.2021.109570
19.) Chen, et al. Co-encapsulation of chlorin e6 and chemotherapeutic drugs in a pegylated liposome enhance the efficacy of tumor treatment: Pharmacokinetics and therapeutic efficacy. Pharmaceutics, 2019, vol. 11, # 11. https://doi.org/10.3390/pharmaceutics11110617
20.) Kim, et al. A novel therapeutic strategy of multimodal nanoconjugates for state-of-the-art brain tumor phototherapy. Journal of Nanobiotechnology, 2022, vol. 20, # 1, art. no. 14. https://doi.org/10.1186/s12951-021-01220-9
21.) Yuan, et al. Direct oligonucleotide-photosensitizer conjugates for photochemical delivery of antisense oligonucleotides. Chemical Communications, 2015, vol. 51, # 30, p. 6678 – 6680. https://doi.org/10.1039/C5CC00573F
22.) Tian, et al. Periodic mesoporous organosilica coupled with chlorin e6 and catalase for enhanced photodynamic therapy to treat triple-negative breast cancer. Journal of Colloid and Interface Science, 2022, vol. 610, p. 634 – 642. https://doi.org/10.1016/j.jcis.2021.11.107