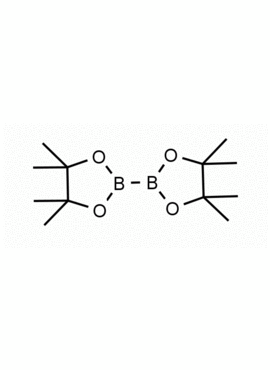
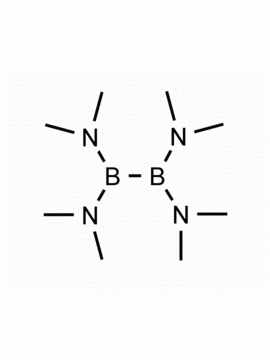
Bis(pinacolato)diboron 4,4,4′,4′,5,5,5′,5′-Octamethyl-2,2′-bi(1,3,2-dioxaborolane) CAS: 73183-34-3 MDL: MFCD00799570
Field of Interest: Organoboron Synthesis, Iridium catalyzed reactions, Transition Metal Reactions,
Background: Bis(pinacolato)diboron is a versatile reagent and specialty chemical that uses Ir-catalyzed, or other transition metals for the C-H borylation of arenes, heteroarenes, and benzylic positions of alkylarenes or pinacolborane furnished aryl-, heteroaryl-, and benzylboron compounds 1,2 This reagent can react with many different functionals and scaffolds under metal catalyzed reactions, including polymers or terminal alkenes to generate diols. 3,4
Molecular weight: 253.94 g/mol
Molecular Formula: C12H24B2O4
CAS Number: 73183-34-3
Storage: Store at–20 °C, under dry conditions.
Synonyms: Bis(pinacolato)diboron, 73183-34-3, bis(pinacolato)diborane, 4,4,4′,4′,5,5,5′,5′, Octamethyl-2,2′-bi(1,3,2-dioxaborolane), Pinacol diborane, 4,4,4′,4′,5,5,5′,5′-Octamethyl-2,2′-bi-1,3,2-dioxaborolane, Diboron pinacol ester, B2Pin2
Selected Synthesis References:
1.) A stoichiometric aromatic C-H borylation catalyzed by iridium(i)/2,2′-bipyridine complexes at room temperature, Ishiyama, Tatsuo; Takagi, Jun; Hartwig, John F.; Miyaura, Norio, Angewandte Chemie, International Edition (2002), 41(16), 3056-3058.
2.) Ambient-Temperature Isolation of a Compound with a Boron-Boron Triple Bond, Braunschweig, Holger; Dewhurst, Rian D.; Hammond, Kai; Mies, Jan; Radacki, Krzysztof; Vargas, Alfredo, Science (Washington, DC, United States) (2012), 336(6087), 1420-1422.
3.) Metal-catalyzed reactions of diborons for synthesis of organoboron compounds, Ishiyama, Tatsuo; Miyaura, Norio, Chemical Record (2004), 3(5), 271-280. DOI:10.1002/tcr.10068
4.) Chemistry of Group 13 element-transition metal linkage – the platinum- and palladium-catalyzed reactions of (alkoxo)diborons, Ishiyama, Tatsuo; Miyaura, Norio, Journal of Organometallic Chemistry (2000), 611(1-2), 392-402. DOI:10.1016/S0022-328X(00)00470-8
5.) Stoichiometric and Catalytic B-C Bond Formation from Unactivated Hydrocarbons and Boranes, Iverson, Carl N.; Smith, Milton R., III, Journal of the American Chemical Society (1999), 121(33), 7696-7697. DOI:10.1021/ja991258w
6.) Platinum(0)-Catalyzed Diboration of Alkynes with Tetrakis(alkoxo)diborons: An Efficient and Convenient Approach to cis-Bis(boryl)alkenes, Ishiyama, Tatsuo; Matsuda, Nobuo; Murata, Miki; Ozawa, Fumiyuki; Suzuki, Akira; Miyaura, Norio, Organometallics (1996), 15(2), 713-20. DOI:10.1021/OM950672B
7.) Metal-catalyzed reactions of organoboronic acids and esters, Miyaura, Norio, Bulletin of the Chemical Society of Japan (2008), 81(12), 1535-1553. DOI:10.1246/bcsj.81.1535
8.) Iridium-catalyzed C-H coupling reaction of heteroaromatic compounds with bis(pinacolato)diboron: regioselective synthesis of heteroarylboronates, Takagi, Jun; Sato, Kazuaki; Hartwig, John F.; Ishiyama, Tatsuo; Miyaura, Norio, Tetrahedron Letters (2002), 43(32), 5649-5651. DOI:10.1016/S0040-4039(02)01135-8
9.) Synthesis of pinacol arylboronates via cross-coupling reaction of bis(pinacolato)diboron with chloroarenes catalyzed by palladium(0)-tricyclohexylphosphine complexes, Ishiyama, Tatsuo; Ishida, Kousaku; Miyaura, Norio, Tetrahedron (2001), 57(49), 9813-9816. DOI:10.1016/S0040-4020(01)00998-X
10.) A borylcopper species generated from bis(pinacolato)diboron and its additions to α,β-unsaturated carbonyl compounds and terminal alkynes, Takahashi, K.; Ishiyama, T.; Miyaura, N., Journal of Organometallic Chemistry (2001), 625(1), 47-53. DOI:10.1016/S0022-328X(00)00826-3
References:
1.) Ishiyama, Tatsuo; Miyaura, Norio, Metal-catalyzed reactions of diborons for synthesis of organoboron compounds, Chemical Record (2004), 3(5), 271-280. DOI:10.1002/tcr.10068.
2.) Billingsley, Kelvin L.; Barder, Timothy E.; Buchwald, Stephen L., Palladium-catalyzed borylation of aryl chlorides: scope, applications, and computational studies, Angewandte Chemie, International Edition (2007), 46(28), 5359-5363. DOI:10.1002/anie.200701551.
3.) Jo, Tae Soo; Kim, Se Hye; Shin, Jihoon; Bae, Chulsung, Highly Efficient Incorporation of Functional Groups into Aromatic Main-Chain Polymer Using Iridium-Catalyzed C-H Activation and Suzuki-Miyaura Reaction, Journal of the American Chemical Society (2009), 131(5), 1656-1657, DOI:10.1021/ja808374e.
4.) Toribatake, Kenji; Nishiyama, Hisao, Asymmetric Diboration of Terminal Alkenes with Rhodium Catalyst and Subsequent Oxidation: Enantioselective Synthesis of Optically Active 1,2-Diols, Angewandte Chemie, International Edition (2013), 52(42), 11011-11015. DOI:10.1002/anie.201305181