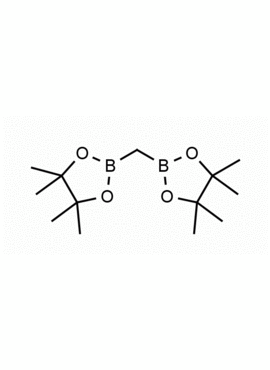
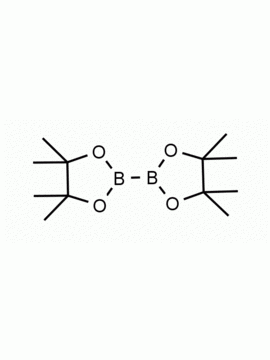
Bis[(pinacolato)boryl]methane, Methylenebis(boronic acid pinacol ester) CAS: 78782-17-9 MDL: MFCD27977747
Sizes Available: 1 g, 5 g, 25 g and larger sizes available
Molecular weight: 267.97 g/mol
Molecular Formula: C13H26B2O4
CAS Number: CAS: 78782-17-9
Storage: Store at –20 °C, under dry conditions.
Synonyms: 78782-17-9, Bis[(pinacolato)boryl]methane, Bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)methane,. 4,4,5,5-tetramethyl-2-[(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)methyl]-1,3,2-dioxaborolane, Methylenedi(boronic Acid Pinacol Ester), Bis((pinacolato)boryl)methane, 2,2′-Methylenebis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane), 2,2′-Methylenebis[4,4,5,5-tetramethyl-1,3,2-dioxaborolane], SCHEMBL10001281
Field of Interest: Transition metal catalyzed cross coupling, organic synthesis, drug discovery
Selected Reactions of Bis[(pinacolato)boryl]methane
1.) Addition Reactions of Bis(pinacolato)diborane(4) to Carbonyl Enones and Synthesis of (pinacolato)2BCH2B and (pinacolato)2BCH2CH2B Insertion and Coupling, Ali, Hijazi Abu Goldberg, Israel Srebnik, Morris, Organometallics (2001), 20(18), 3962-3965. DOI:10.1021/om010282r
2.) BH Bond Activation of Trimethylphosphineborane Transition Metal Complexes: Synthesis of Metal Complexes Bearing Nonsubstituted Boryl-Trimethylphosphine, Cp*M(CO)3(BH2·PMe3) (M = Mo, Kawano, Yasuro Yasue, Takahiro Shimoi, Mamoru, Journal of the American Chemical Society (1999), 121(50), 11744-11750. DOI:10.1021/ja992168u
3.) Silver-Assisted, Iridium-Catalyzed Allylation of Bis[(pinacolato)boryl]methane Allows the Synthesis of Enantioenriched Homoallylic Organoboronic Esters, Zhan, Miao Li, Ren-Zhe Mou, Ze-Dong Cao, Chao-Guo Liu, Jie Chen, Yuan-Wei Niu, Dawen, ACS Catalysis (2016), 6(5), 3381-3386. DOI:10.1021/acscatal.6b00719
4.) Understanding the Reactivity Difference of Metal Boryl Complexes toward Alkanes and Arenes: A Density Functional Study on the Functionalizations of Methane and Benzene CpM(CO)n(BO2C2H2) (M = Fe, Ru, W), Lam, Wai Han Lin, Zhenyang, Organometallics (2003), 22(3), 473-480. DOI:10.1021/om020901b
5.) Enantioselective Synthesis of (E)-δ-Boryl-Substituted anti-Homoallylic Alcohols Using Palladium and a Chiral Phosphoric Acid, Miura, Tomoya Nakahashi, Junki Murakami, Masahiro, Angewandte Chemie, International Edition (2017), 56(24), 6989-6993. DOI:10.1002/anie.201702611
6.) Chemistry of polynuclear metal complexes with bridging carbene or car ne ligands. Part 108. Synthesis and reactions of the alkylidynetungsten complexes [W(≡CR)(CO)2{(F3B)C(pz)3}] (R = Me or C6H4Me-4, pz = pyrazol-1-yl) and their use as reagents for preparing di- and tri-metal compounds, Byers, Peter K. Stone, F. Gordon A., Journal of the Chemical Society, Dalton Transactions: Inorganic Chemistry (1972-1999) (1991), (1), 93-9. Language: English, Database: CAPLUS
7.) Synthesis of E- and Z-trisubstituted alkenes catalytic cross-metathesis, Nguyen, Thach T. Koh, Ming Joo Mann, Tyler J. Schrock, Richard R. Hoveyda, Amir H., Nature (London, United Kingdom) (2017), 552(7685), 347-354. DOI:10.1038/nature25002
8.) gem-Silylborylation approach for tri- and tetrametalmethanes: the first synthesis of boryl(germyl)(silyl)(stannyl)methanes, Shimizu, Masaki Kurahashi, Takuya Kitagawa, Hirotaka Shimono, Katsuhiro Hiyama, Tamejiro, Journal of Organometallic Chemistry (2003), 686(1-2), 286-293. DOI:10.1016/S0022-328X(03)00561-8
9.) Metal-Organic Framework Stabilizes a Low-Coordinate Iridium Complex for Catalytic Methane Borylation, Feng, Xuanyu Song, Yang Li, Zhe Kaufmann, Michael Pi, Yunhong Chen, Justin S. Xu, Ziwan Li, Zhong Wang, Cheng Lin, Wenbin, Journal of the American Chemical Society (2019), 141(28), 11196-11203. DOI:10.1021/jacs.9b04285
10.) Esters as Radical Acceptors: β-NHC-Borylalkenyl Radicals Induce Lactonization C-C Bond Formation/Cleavage on Esters, Shimoi, Masaki Maeda, Katsuhiro Geib, Steven J. Curran, Dennis P. Taniguchi, Tsuyoshi, Angewandte Chemie, International Edition (2019), 58(19), 6357-6361. DOI:10.1002/anie.201902001
11.) Enantiodivergent Synthesis of Allenes Point-to-Axial Chirality Transfer, Armstrong, Roly J. Nandakumar, Meganathan Dias, Rafael M. P. Noble, Adam Myers, Eddie L. Aggarwal, Varinder K., Angewandte Chemie, International Edition (2018), 57(27), 8203-8208. DOI:10.1002/anie.201804446
12.) Reactivity of the geminal phosphinoborane tBu2PCH2BPh2 towards alkynes, nitriles, and nitrilium triflates, Habraken, Evi R. M. Mens, Lars C. Nieger, Martin Lutz, Martin Ehlers, Andreas W. Slootweg, J. Chris, Dalton Transactions (2017), 46(36), 12284-12292. DOI:10.1039/C7DT02570J
13.) Palladium-catalyzed oxidative allylation of bis[(pinacolato)boryl]methane: synthesis of homoallylic boronic esters, Li, Chunsheng Li, Meng Li, Jianxiao Wu, Wanqing Jiang, HuanfenChemical Cog mmunications (Cambridge, United Kingdom) (2018), 54(1), 66-69. DOI:10.1039/C7CC07788B