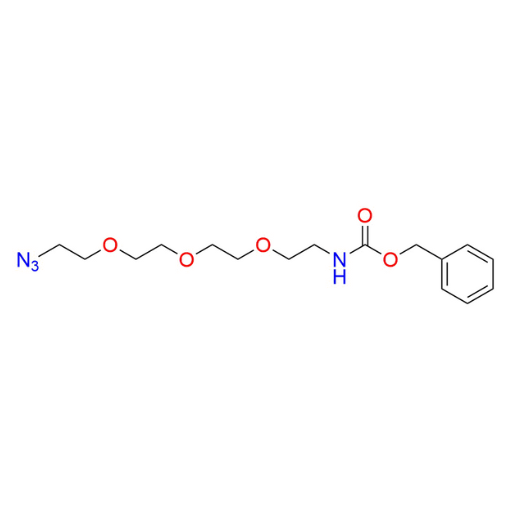
Name: Benzyl (2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethyl)carbamate
Molecular Formula: C16H24N4O5
CAS#: 863973-27-7
SMILES: O=C(OCC1=CC=CC=C1)NCCOCCOCCOCCN=[N+]=[N-]
MDL#: AMTGC1389-BA24
Catalog#: MFCD35646360
Molecular weight: 352.39 g/mol
Other names:
- 5,8,11-TRIOXA-2-AZATRIDECANOIC ACID, 13-AZIDO-, PHENYLMETHYL ESTER
- 863973-27-7
Fields of Interest: PEGylation, bioconjugation, drug delivery, materials science, click chemistry
Background & Applications:
Background
Benzyl (2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethyl)carbamate (CAS 863973-27-7) is a protected azide-functionalized PEG derivative based on a PEG4 backbone, featuring a terminal azide group and a benzyl carbamate (Cbz-protected) primary amine. The polyethylene glycol spacer provides hydrophilicity, flexibility, and compatibility with aqueous and biological environments, while the Cbz protecting group enables controlled, stepwise synthetic strategies by temporarily masking amine reactivity. The azide functionality supports efficient bioorthogonal click chemistry, including CuAAC and strain-promoted azide–alkyne cycloaddition. This compound serves as a versatile intermediate within a robust portfolio of functionalized PEGs designed for precise molecular assembly.
Applications
This Cbz-protected azido PEG linker is commonly used in bioconjugation, drug delivery, and materials science applications where orthogonal reactivity and staged functional group exposure are required. Typical uses include stepwise construction of multifunctional PEG linkers, selective PEGylation of small molecules or polymers, and preparation of intermediates for pharmaceutical and biomaterials research. Following hydrogenolytic deprotection of the benzyl carbamate, the resulting azido–amine PEG is suitable for direct coupling to activated carboxylic acids, NHS esters, and isocyanates. As part of a comprehensive functionalized PEG product line, Benzyl (2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethyl)carbamate supports modular design strategies for advanced chemical and biological applications.
Appearance: Pale yellow liquid
Purity: 99%
Storage: 0-3 °C for long term storage
Solubility: DCM, Chloroform, Ethyl Acetate, Acetonitrile
Literature:
Cbz-protected amino-PEG linkers are well-established intermediates in bioconjugation, drug delivery, and polymer–drug conjugate research, where controlled and orthogonal amine protection is required during multistep synthesis. These compounds combine the hydrophilicity and flexibility of a polyethylene glycol (PEG) spacer with a carboxybenzyl (Cbz)–protected amine, allowing selective deprotection by catalytic hydrogenolysis at a late synthetic stage.
In the peer-reviewed literature, Cbz-protected PEG amines are frequently employed as enabling building blocks for the construction of PEGylated small molecules, biomaterials, and antibody–drug conjugates, providing improved solubility, reduced nonspecific interactions, and precise control over linker architecture.
Their role in modern conjugate design is discussed in broader reviews of PEG-based linker strategies, such as the Journal of Controlled Release review on polyethylene glycol linkers in advanced drug conjugates: https://www.sciencedirect.com/science/article/pii/S0168365921003849.