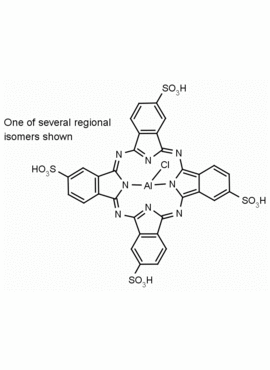
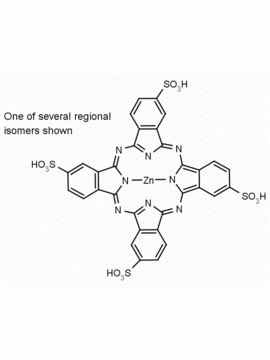
Al(III) Phthalocyanine Chloride Tetrasulfonic Acid CAS Number: 100180-30-1 MDL: MFCD00672294
Molecular weight: 95.21 g/mol
Molecular Formula: C32H16AlClN8O12S4
CAS Number: 100180-30-1
Storage: Store at room temperature, protect from light
Synonyms: AL(III) PHTHALOCYANINE CHLORIDE TETRASULFONIC ACID, Aluminum, chloro[29
Fields of Interest: Photodynamic Therapy, Nanocomposites, Quantum Oxygen Yield, Catalysis, Photocatalysis
Background: Al(III) Phthalocyanine Chloride Tetrasulfonic Acid is a phthalocyanine specialty chemical manufactured by Frontier Specialty Chemicals.
References:
1.) Wang D, Huang J, Li X, Yang P, Du Y, et al. 2015. Photocatalytic H2 production under visible-light irradiation based on covalent attachment of manganese phthalocyanine to graphene. J. Mater. Chem. A 3:4195-202
2.) Villemin D, Hammadi M, Hachemi M, Bar N. 2001. Applications of microwave in organic synthesis: an improved one-step synthesis of metallophthalocyanines and a new modified microwave oven for dry reactions. Molecules 6:831-44
3.) Tarasevich MR, Zhutaeva GV, Bogdanovskaya VA, Radina MV, Ehrenburg MR, Chalykh AE. 2007. Oxygen kinetics and mechanism at electrocatalysts on the base of palladium-iron system. Electrochim. Acta 52:5108-18
4.) Spiller W, Kliesch H, Wohrle D, Hackbarth S, Roder B, Schnurpfeil G. 1998. Singlet oxygen quantum yields of different photosensitizers in polar solvents and micellar solutions. J. Porphyrins Phthalocyanines 2:145-58
5.) Sommerauer M, Rager C, Hanack M. 1996. Separation of 2(3),9(10),16(17),23(24)-Tetrasubstituted Phthalocyanines with Newly Developed HPLC Phases. J. Am. Chem. Soc. 118:10085-93
6.) Shaabani A, Farhangi E, Rahmati A. 2008. Aerobic oxidation of alkyl arenes and alcohols using cobalt(II) phthalocyanine as a catalyst in 1-butyl-3-methyl-imidazolium bromide. Appl. Catal., A 338:14-9
7.) Ribeiro AO, Tome JPC, Neves MGPMS, Tome AC, Cavaleiro JAS, et al. 2006. [1,2,3,4-Tetrakis(α/β-D-galactopyranos-6-yl)phthalocyaninato]zinc(II): a water-soluble phthalocyanine. Tetrahedron Lett. 47:9177-80
8.) Plaetzer K, Kiesslich T, Krammer B, Hammerl P. 2002. Characterization of the cell death modes and the associated changes in cellular energy supply in response to AlPcS4-PDT. Photochem. Photobiol. Sci. 1:172-7
9.) Parton RF, Vankelecom IFJ, Tas D, Janssen KBM, Knops-Gerrits P-P, Jacobs PA. 1996. Membrane occluded catalysts: a higher order mimic with improved performance. J. Mol. Catal. A: Chem. 113:283-92
10.) Paradine SM, White MC. 2012. Iron-Catalyzed Intramolecular Allylic C-H Amination. J. Am. Chem. Soc. 134:2036-9
11.) Luo G-F, Chen W-H, Lei Q, Qiu W-X, Liu Y-X, et al. 2016. A Triple-Collaborative Strategy for High-Performance Tumor Therapy by Multifunctional Mesoporous Silica-Coated Gold Nanorods. Adv. Funct. Mater. 26:4339-50
12.) Liang X, Chen Z, Wu H, Guo L, He C, et al. 2014. Enhanced NH3-sensing behavior of 2,9,16,23-tetrakis(2,2,3,3-tetrafluoropropoxy) metal(II) phthalocyanine/multi-walled carbon nanotube hybrids: An investigation of the effects of central metals. Carbon 80:268-78
13.) Iliev V, Mihaylova A, Bilyarska L. 2002. Photooxidation of phenols in aqueous solution, catalyzed by mononuclear and polynuclear metal phthalocyanine complexes. J. Mol. Catal. A: Chem. 184:121-30
14.) Hassan SSM, Mahmoud WH, Elmosallamy MAF, Almarzooqi MH. 2005. Iron(II)-phthalocyanine as a novel recognition sensor for selective potentiometric determination of diclofenac and warfarin drugs. J. Pharm. Biomed. Anal. 39:315-21
15.) Durmus M, Nyokong T. 2007. Synthesis, photophysical and photochemical properties of aryloxy tetra-substituted gallium and indium phthalocyanine derivatives. Tetrahedron 63:1385-94
16.) Drechsler U, Pfaff M, Hanack M. 1999. Synthesis of novel functionalised zinc phthalocyanines applicable in photodynamic therapy. Eur. J. Org. Chem.:3441-53
17.) Dhami S, Cosa JJ, Bishop SM, Phillips D. 1996. Photophysical Characterization of Sulfonated Aluminum Phthalocyanines in a Cationic Reversed Micellar System. Langmuir 12:293-300
18.) Cissell JA, Vaid TP, Rheingold AL. 2006. Aluminum Tetraphenylporphyrin and Aluminum Phthalocyanine Neutral Radicals. Inorg. Chem. 45:2367-9
19.) Cho SW, Piper LFJ, De Masi A, Preston ARH, Smith KE, et al. 2010. Electronic Structure of C60/Phthalocyanine/ITO Interfaces Studied using Soft X-ray Spectroscopies. J. Phys. Chem. C 114:1928-33
20.) Cheng H, Zhu J-Y, Li S-Y, Zeng J-Y, Lei Q, et al. 2016. An O2 Self-Sufficient Biomimetic Nanoplatform for Highly Specific and Efficient Photodynamic Therapy. Adv. Funct. Mater. 26:7847-60
21.) Chen H, Xiao L, Anraku Y, Mi P, Liu X, et al. 2014. Polyion Complex Vesicles for Photoinduced Intracellular Delivery of Amphiphilic Photosensitizer. J. Am. Chem. Soc. 136:157-63
22.) Chauke V, Ogunsipe A, Durmus M, Nyokong T. 2007. Novel gallium(III) phthalocyanine derivatives – Synthesis, photophysics and photochemistry. Polyhedron 26:2663-71
23.) Brunel M, Chaput F, Vinogradov SA, Campagne B, Canva M, et al. 1997. Reverse saturable absorption in palladium and zinc tetraphenyltetrabenzoporphyrin doped xerogels. Chem. Phys. 218:301-7
24.) Berlanda J, Kiesslich T, Engelhardt V, Krammer B, Plaetzer K. 2010. Comparative in vitro study on the characteristics of different photosensitizers employed in PDT. J. Photochem. Photobiol., B 100:173-80
25.) Baker R, Wilkinson DP, Zhang J. 2008. Electrocatalytic activity and stability of substituted iron phthalocyanines towards oxygen reduction evaluated at different temperatures. Electrochim. Acta 53:6906-19