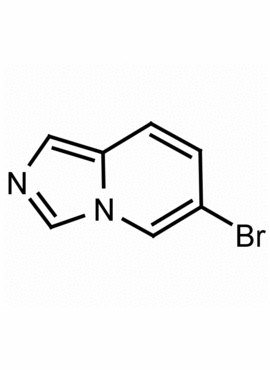
6-Bromoimidazo[1,5-a]pyridine CAS: 1239880-00-2 MDL: MFCD17677174
Molecular weight: 197.03 g/mol
Molecular Formula: C7H5BrN2
CAS Number: 1239880-00-2
Storage: Store at 2-8 Co, under dry conditions.
Synonyms: 6-bromoimidazo[1,5-a]pyridine, imidazo[1,5-a]pyridine, 6-bromo-
Uses: Synthesis building block, Organic Synthesis, imidazole-pyridine nitrogen heterocycle, synthesis, bromine reactions
6-Bromoimidazo[1,5-a]pyridine, is a synthetic fine chemical useful in the synthesis of pharmaceuticals and fine organic chemicals.
Selected References:
- Albaugh, P. A.; Choi, H.-S.; Chopiuk, G.; Fan, Y.; Rucker, P. V.; Wang, Z. Preparation of imidazopyridazines and similar compounds as therapeutic kinase inhibitors. WO2009140128A2, 2009.
- Almirante, L.; Mugnaini, A.; De Toma, N.; Gamba, A.; Murmann, W.; Hidalgo, J., Imidazole derivatives. IV. Synthesis and pharmacologic activity of oxygenated derivatives of imidazo[1,2-a]pyridine. J. Med. Chem. 1970, 13 (6), 1048-51.
- Bruice, T. C.; Schmir, G. L., Imidazole catalysis. II. The reaction of substituted imidazoles with phenyl acetates in aqueous solution. J. Am. Chem. Soc. 1958, 80, 148-56.
- Gudmundsson, K.; Boggs, S. D. Preparation of imidazo[1,2-a]pyridine derivatives as chemokine receptor ligands with anti-HIV activity. WO2006026703A2, 2006.
- Ismail, M. A.; Arafa, R. K.; Wenzler, T.; Brun, R.; Tanious, F. A.; Wilson, W. D.; Boykin, D. W., Synthesis and antiprotozoal activity of novel bis-benzamidino imidazo[1,2-a]pyridines and 5,6,7,8-tetrahydro-imidazo[1,2-a]pyridines. Bioorg. Med. Chem. 2008, 16 (2), 683-691.
- Ni, Z.-J.; Pecchi, S.; Burger, M.; Han, W.; Smith, A.; Atallah, G.; Bartulis, S.; Frazier, K.; Verhagen, J.; Zhang, Y.; Iwanowicz, E.; Hendrickson, T.; Knapp, M.; Merritt, H.; Voliva, C.; Wiesmann, M.; Legrand, D. M.; Bruce, I.; Dale, J.; Lan, J.; Levine, B.; Costales, A.; Liu, J.; Pick, T.; Menezes, D. Preparation of imidazo[1,2-a]pyridines and imidazo[1,2-b]pyridazines as PI-3 kinase inhibitors. WO2007095588A1, 2007.
- Ratcliffe, A. J.; Alam, M.; Beevers, R. E.; Davenport, R. J.; Davies, N.; Haughan, A. F.; Jones, M. W.; Lowe, C.; Perry, B. G.; Phillips, D. J.; Pitt, W. R.; Sharpe, A. Preparation of aminopyrimidines as JNK inhibitors. WO2006038001A1, 2006.
- Ratni, H.; Karp, G. M.; Weetall, M.; Naryshkin, N. A.; Paushkin, S. V.; Chen, K. S.; McCarthy, K. D.; Qi, H.; Turpoff, A.; Woll, M. G.; Zhang, X.; Zhang, N.; Yang, T.; Dakka, A.; Vazirani, P.; Zhao, X.; Pinard, E.; Green, L.; David-Pierson, P.; Tuerck, D.; Poirier, A.; Muster, W.; Kirchner, S.; Mueller, L.; Gerlach, I.; Metzger, F., Specific Correction of Alternative Survival Motor Neuron 2 Splicing by Small Molecules: Discovery of a Potential Novel Medicine To Treat Spinal Muscular Atrophy. J. Med. Chem. 2016, 59 (13), 6086-6100.
- Uchikawa, O.; Sakai, N.; Terao, Y.; Suzuki, H. Preparation of fused heterocyclic compounds as apoptosis signal regulating kinase 1 (ASK1) inhibitors. WO2008016131A1, 2008.
- Zhou, X.; Yan, H.; Ma, C.; He, Y.; Li, Y.; Cao, J.; Yan, R.; Huang, G., Copper-Mediated Aerobic Oxidative Synthesis of 3-Bromo-imidazo[1,2-a]pyridines with Pyridines and Enamides. J. Org. Chem. 2016, 81 (1), 25-31.