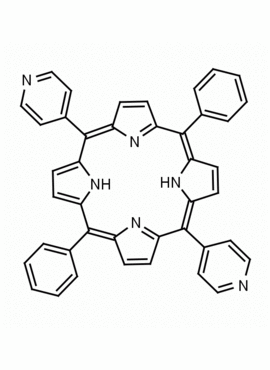
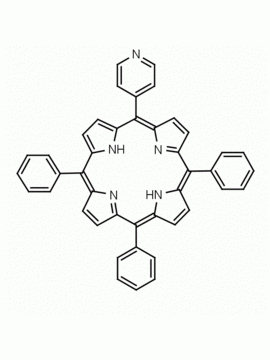
5,15-Diphenyl-10,20-di(4-pyridyl)-21H,23H-porphine CAS: 71410-72-5 MDL: MFCD16628185
Molecular weight: 616.712 g/mol
Molecular Formula: C42H28N6
CAS Number: 71410-72-5
Storage: Store at room temperature, protect from light.
Synonyms: 21H,23H-Porphine, 5
Fields of Interest: Synthetic Porphyrins, Metal Organic Frameworks (MOFs), Supramolecular Chemistry, Self-Assembly
Background: 5,15-Diphenyl-10,20-di(4-pyridyl)-21H,23H-porphine is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals. 5,15-Diphenyl-10,20-di(4-pyridyl)-21H,23H-porphine is of interest as a bifunctional porphyrin based linker for supramolecular chemistry.
References:
1.) Drain, et al. Self-assembly of square multiporphyrin arrays by metal ion coordination. J. Chem. Soc., Chem. Commun., 1994, 2313-2315. https://doi.org/10.1039/C39940002313
2.) Zimmerman, et al. Molecular Imprinting Inside Dendrimers. J. Am. Chem. Soc. 2003, 125, 44, 13504–13518. https://doi.org/10.1021/ja0357240
3.) Deiters, et al. Reversible single-crystal-to-single-crystal guest exchange in a 3-D coordination network based on a zinc porphyrin. Chem. Commun., 2005, 3906-3908. https://doi.org/10.1039/B508135C
4.) Rucareanu, et al. Supramolecular Assemblies between Macrocyclic Porphyrin Hexamers and Star-Shaped Porphyrin Arrays. J. Org. Chem. 2001, 66, 15, 4973–4988. https://doi.org/10.1021/jo0015523
5.) Cai, et al. Binding of Dual-Function Hybridized Metal–Organic Capsules to Enzymes for Cascade Catalysis. JACS Au 2022, 2, 7, 1736–1746. https://doi.org/10.1021/jacsau.2c00322
6.) Zhang, et al. Platinum(II) Metallatriangle: Construction, Coassembly with Polypeptide, and Application in Combined Cancer Photodynamic and Chemotherapy. Inorg. Chem. 2021, 60, 11, 7627–7631. https://doi.org/10.1021/acs.inorgchem.1c00962
7.) Vidal. et al. A Flexible Synthetic Strategy for the Preparation of Heteroleptic Metallacycles of Porphyrins. Inorg. Chem. 2021, 60, 15, 11503–11513. https://doi.org/10.1021/acs.inorgchem.1c01511
8.) Yao, et al. 2D amphiphilic organoplatinum(ii) metallacycles: their syntheses, self-assembly in water and potential application in photodynamic therapy. Chem. Commun., 2018,54, 8068-8071. https://doi.org/10.1039/C8CC04423F