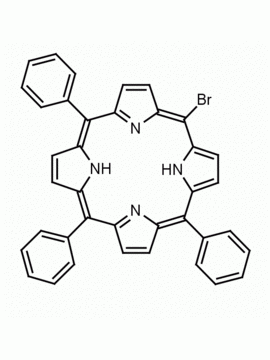
5,15-dibromo-10,20-diphenylporphine CAS: 151256-86-9 MDL: MFCD09260447
Molecular weight: 620.336 g/mol
Molecular Formula: C32H20Br2N4
CAS Number: 151256-86-9
Storage: Store at room temperature, protect from light.
Synonyms: 151256-86-9, 21H,23H-Porphine, 5
Fields of Interest: Synthetic Porphyrins, Functionalized Porphyrins
Background: 5,15-dibromo-10,20-diphenylporphine is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals. 5,15-dibromo-10,20-diphenylporphine is useful for synthesizing highly functionalized porphyrins by cross coupling chemistry.
References:
1.) Son, et al. Light-Harvesting and Ultrafast Energy Migration in Porphyrin-Based Metal–Organic Frameworks. J. Am. Chem. Soc. 2013, 135, 2, 862–869. https://doi.org/10.1021/ja310596a
2.) DiMagno, et al. Facile elaboration of porphyrins via metal-mediated cross-coupling. J. Org. Chem. 1993, 58, 22, 5983–5993. https://doi.org/10.1021/jo00074a027
3.) Chen, et al. Facile and Efficient Synthesis of meso-Arylamino- and Alkylamino-Substituted Porphyrins via Palladium-Catalyzed Amination. J. Org. Chem. 2003, 68, 11, 4432–4438. https://doi.org/10.1021/jo034063m
4.) Aratani, et al. Synthesis of meso-meso Linked Hybrid Porphyrin Arrays by Pd-Catalyzed Cross-Coupling Reaction. Org. Lett. 2001, 3, 26, 4213–4216. https://doi.org/10.1021/ol0168770
5.) Gao, et al. Versatile Synthesis of meso-Aryloxy- and Alkoxy-Substituted Porphyrins via Palladium-Catalyzed C−O Cross-Coupling Reactions. Org. Lett. 2003, 5, 18, 3261–3264. https://doi.org/10.1021/ol035081t
6.) Gao, et al. Synthesis of meso-Arylsulfanyl- and Alkylsulfanyl-Substituted Porphyrins via Palladium-Mediated C−S Bond Formation. J. Org. Chem. 2004, 69, 25, 8886–8892. https://doi.org/10.1021/jo048552d
7.) Gao, et al. General Synthesis of meso-Amidoporphyrins via Palladium-Catalyzed Amidation. Org. Lett. 2004, 6, 11, 1837–1840. https://doi.org/10.1021/ol049440b
8.) Vaz, et al. Suzuki cross-coupling of meso-dibromoporphyrins for the synthesis of functionalized A2B2 porphyrins. Tetrahedron Letters. Volume 42, Issue 42, 15 October 2001, Pages 7409-7412. https://doi.org/10.1016/S0040-4039(01)01582-9