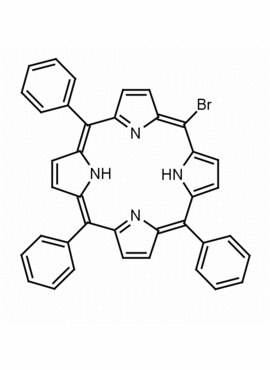
5-Monobromo-10,15,20-triphenylporphine, 5-Bromo-10,15,20-triphenyl-21H,23H-porphine CAS: 67066-09-5 MDL: MFCD12022335
Molecular weight: 617.536 g/mol
Molecular Formula: C38H25BrN4
CAS Number: 67066-09-5
Storage: Store at room temperature, protect from light.
Synonyms: 21H,23H-Porphine, 5
Fields of Interest: Synthetic Porphyrins, Mono Functionalized Porphyrins, Porphyrin Dimers
Background: 5-Monobromo-10,15,20-triphenylporphine is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals. 5-Monobromo-10,15,20-triphenylporphine is a useful starting material for the construction of functionalized porphyrins by transition metal mediated cross coupling.
References:
1.) Hisano, et al. Host–Guest Complexation of Bisporphyrin Cleft and Electron-Deficient Aromatic Guests. J. Org. Chem. 2022, 87, 6, 4001–4009. https://doi.org/10.1021/acs.joc.1c02742
2.) Schissler, et al. A Synthetic Strategy for Cofacial Porphyrin-Based Homo- and Heterobimetallic Complexes. Chemistry, A European Journal. Volume 27, Issue 9, February 10, 2021, Pages 3047-3054. https://doi.org/10.1002/chem.202002394
3.) Stefan, et al. Cubane Cross-Coupling and Cubane–Porphyrin Arrays. Chemistry, A European Journal. Volume 24, Issue 5, January 24, 2018, Pages 1026-1030. https://doi.org/10.1002/chem.201704344
4.) Plunkett, et al. Synthesis and Reactivity of Allenylporphyrins. EurJOC. Volume 2013, Issue 8, March 2013, Pages 1566-1579. https://doi.org/10.1002/ejoc.201201535
5.) Locos, et al. Homo- and Heteronuclear meso,meso-(E)-Ethene-1,2-diyl-Linked Diporphyrins: Preparation, X-ray Crystal Structure, Electronic Absorption and Emission Spectra and Density Functional Theory Calculations. Chemistry, A European Journal. Volume 18, Issue 18, April 27, 2012, Pages 5574-5588.
6.) Sugita, et al. Palladium-catalyzed Kumada Coupling Reaction of Bromoporphyrins with Silylmethyl Grignard Reagents: Preparation of Silylmethyl-substituted Porphyrins as a Multipurpose Synthon for Fabrication of Porphyrin Systems. J. Org. Chem. 2012, 77, 23, 10488–10497. https://doi.org/10.1021/jo302122f
7.) Ryan, et al. Porphyrin Dimers and Arrays, EurJOC. Volume 2011, Issue 29, October 2011, Pages 5817-5844. https://doi.org/10.1002/ejoc.201100642
8.) Locos, The Heck reaction for porphyrin functionalisation: synthesis of meso-alkenyl monoporphyrins and palladium-catalysed formation of unprecedented meso–β ethene-linked diporphyrins. Org. Biomol. Chem., 2006,004, 902-916. https://doi.org/10.1039/B516989E
9.) Takanami, et al. Palladium-Catalyzed Cyanation of Porphyrins Utilizing Cyanoethylzinc Bromide as an Efficient Cyanide Ion Source. Org. Lett. 2005, 7, 18, 3937–3940. https://doi.org/10.1021/ol0514294
10.) Oike, et al. Polyether-Bridged Sexithiophene as a Complexation-Gated Molecular Wire for Intramolecular Photoinduced Electron Transfer. J. Am. Chem. Soc. 2005, 127, 44, 15372–15373. https://doi.org/10.1021/ja055648w