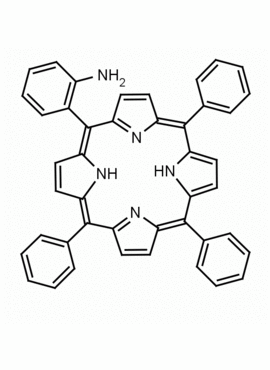
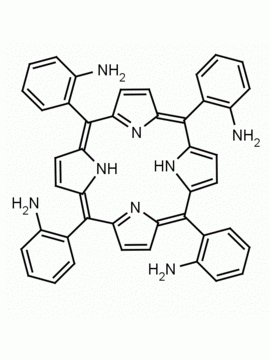
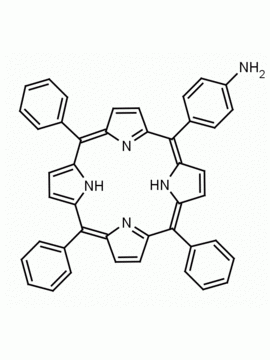
5-mono(2-aminophenyl)-10,15,20-triphenyl porphine CAS: 69082-94-6 MDL:
Molecular weight: 629.75 g/mol
Molecular Formula: C44H31N5
CAS Number: 69082-94-6
Storage: Store at room temperature, protect from light.
Synonyms: 5-mono(2-aminophenyl)-10,15,20-triphenyl porphine, 69082-94-6
Fields of Interest: Synthetic Porphyrins, Mono Functionalized Porphyrins, Catalysis, Second Sphere Catalysts
Background: 5-mono(2-aminophenyl)-10,15,20-triphenyl porphine is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals. 5-mono(2-aminophenyl)-10,15,20-triphenyl porphine is useful as a ligand for studying second sphere coordination reactivity.1-6
References:
1.) Ghatak, et al. Second-Sphere Hydrogen-Bond Donors and Acceptors Affect the Rate and Selectivity of Electrochemical Oxygen Reduction by Iron Porphyrins Differently. Inorg. Chem. 2022, 61, 33, 12931–12947. https://doi.org/10.1021/acs.inorgchem.2c02170
2.) Nichols, et al. Positional effects of second-sphere amide pendants on electrochemical CO2 reduction catalyzed by iron porphyrins. Chem. Sci., 2018,9, 2952-2960. https://doi.org/10.1039/C7SC04682K
3.) Bhunia, et al. Rational Design of Mononuclear Iron Porphyrins for Facile and Selective 4e–/4H+ O2 Reduction: Activation of O–O Bond by 2nd Sphere Hydrogen Bonding. J. Am. Chem. Soc. 2018, 140, 30, 9444–9457. https://doi.org/10.1021/jacs.8b02983
4.) Bhakta, et al. Induction of Enzyme-like Peroxidase Activity in an Iron Porphyrin Complex Using Second Sphere Interactions. Inorg. Chem. 2019, 58, 5, 2954–2964. https://doi.org/10.1021/acs.inorgchem.8b02707
5.) Bhunia, et al. A designed second-sphere hydrogen-bond interaction that critically influences the O–O bond activation for heterolytic cleavage in ferric iron–porphyrin complexes. Chem. Sci., 2020,11, 2681-2695. https://doi.org/10.1039/C9SC04388H
6.) Williams, et al. Atropisomeric Effects of Second Coordination Spheres on Electrocatalytic CO2 Reduction. ChemCatChem. Volume 12, Issue 19, October 6, 2020, Pages 4886-4892.