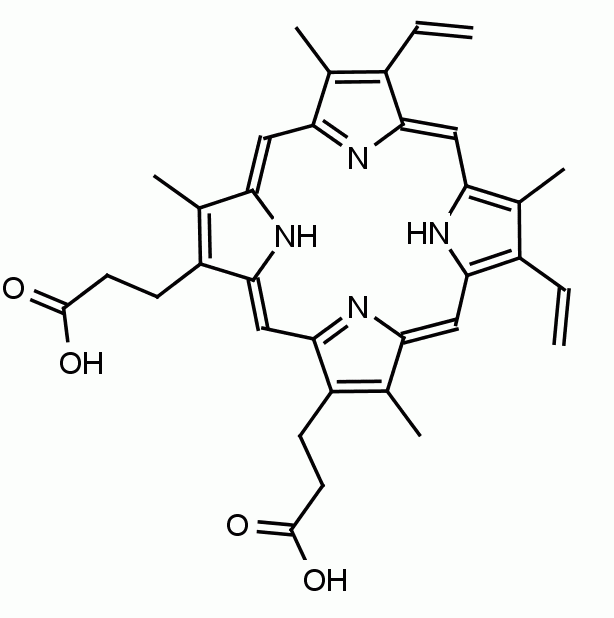
Protoporphyrin IX 8,13-Bis(vinyl)-3,7,12,17-tetramethyl-21H,23H-porphine-2,18-dipropionic acid CAS: 553-12-8 MDL: MFCD00151109
Field of Interest: Heme, Cytochrome C and Chlorophyll Biosynthesis, Inflammation activity, Photosynthesis and Chlorophyll degradation product
Background: Protoporphyrin IX is active as a photosensitizer and is a component of nitric oxide synthase for NOS generation.1, 2 It is a substrate for heme oxygenases affording cellular protection and producing biliverdin and CO, a secondary messenger in cells, and metallated (Fe) versions of heme is one of the most abundant and widely used metalloporphyrins in the biosphere 3,4
Molecular weight: 562.658 g/mol
Molecular Formula: C34H34N4O4
CAS Number: 553-12-8
Storage: Store at–20 °C, under dry conditions.
Synonyms: ChEMBL267548, 2,18-Porphinedipropionic acid, 3,8,13,17-tetramethyl-7,12-divinyl-, 21H,23H-Porphine-2,18-dipropanoic acid, 7,12-diethenyl-3,8,13,17-tetramethyl-, 3,3′-(3,7,12,17-Tetramethyl-8,13-divinylporphin-2,18-diyl)di(propionsaure), 3,3′-(3,7,12,17-tetramethyl-8,13-divinylporphine-2,18-diyl)di(propionic acid), Acide 3,3′-(3,7,12,17-tetramethyl-8,13-divinylporphine-2,18-diyl)dipropionique, acido 3,3′-(3,7,12,17-tetrametil-8,13-divinilporfina-2,18-diil)dipropionico, Kammerer’s porphyrin, NSC 177389, NSC 2632, Ooporphyrin, Protoporphyrin, Protoporphyrin IX
References:
1) Castano, Ana P.; Demidova, Tatiana N.; Hamblin, Michael R., 1Mechanisms in photodynamic therapy: part one-photosensitizers, photochemistry and cellular localization, Photodiagnosis and Photodynamic Therapy (2005), Volume Date2004, 1(4), 279-293. 2) Alderton, Wendy K.; Cooper, Chris E.; Knowles, Richard G., Nitric oxide synthases: structure, function and inhibition, Biochemical Journal (2001), 357(3), 593-615. 3) Gozzelino, Raffaella; Jeney, Viktoria; Soares, Miguel P., Mechanisms of cell protection by heme oxygenase-1, Annual Review of Pharmacology and Toxicology (2010), 50, 323-354. 4) Poulos, Thomas L., Heme Enzyme Structure and Function, Chemical Reviews (Washington, DC, United States) (2014), 114(7), 3919-3962.