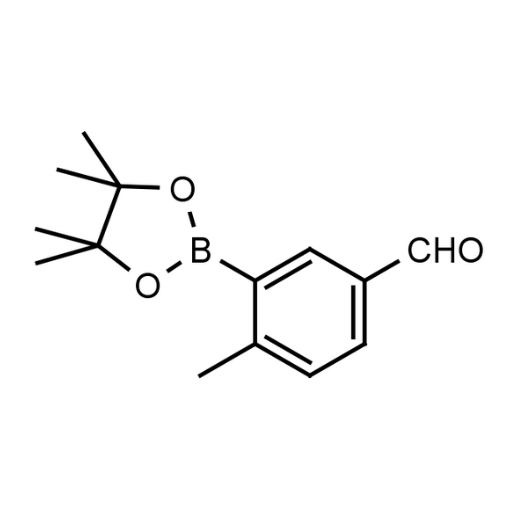
Name: 5-Formyl-2-methylphenylboronic acid pinacol ester
Molecular Formula: C14H19BO3
CAS#: 847560-50-3
SMILES: CC1=CC=C(C=O)C=C1B2OC(C)(C)C(C)(C)O2
MDL#: MFCD18731017
Catalog#: F36226
Molecular weight: 246.11 g/mol
Other names:
- Name 1: (5-Formyl-2-methylphenyl)boronic acid pinacol ester
- Name 2: 4-Methyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzaldehyde
Fields of Interest: Organic synthesis, Suzuki coupling reactions
Background & Applications: 5-Formyl-2-methylphenylboronic acid pinacol ester is an organoboron compound featuring a boronic ester group, a formyl (aldehyde) group, and a methyl substituent on an aromatic ring. The pinacol ester moiety enhances the compound’s stability compared to its corresponding boronic acid, making it more amenable to storage and handling in synthetic applications. However, like other pinacol boronic esters, it remains sensitive to hydrolysis, especially under aqueous conditions, necessitating careful handling during reactions and storage.
Applications:
5-Formyl-2-methylphenylboronic acid pinacol ester serves as a versatile intermediate in organic synthesis. The aldehyde group allows for further functionalization through reactions such as reductive amination or condensation, while the boronic ester group is pivotal in cross-coupling reactions, notably the Suzuki–Miyaura coupling, facilitating the formation of carbon–carbon bonds. Additionally, boronic esters have been employed in the development of fluorescent probes for detecting reactive oxygen species and metal ions in biological systems.
Appearance: White solid
Purity: 98%
Storage: 3-5 °C
Solubility: Ethyl acetate, Dichloromethane, chloroform, Diethyl ether
Literature:
Clausen, F., Kischkewitz, M., Bergander, K., & Studer, A. (2019). Catalytic protodeboronation of pinacol boronic esters: formal anti-Markovnikov hydromethylation of alkenes. Chemical Science, 10(24), 6210–6214. https://doi.org/10.1039/c9sc02067e
Dai, L., Gonzalez, J., & Zhang, K. (2022). A simple generic method for analyzing water sensitive pinacol boronate compounds by hydrophilic interaction liquid chromatography. Journal of Chromatography Open, 2, 100036. https://doi.org/10.1016/j.jcoa.2022.100036
Lennox, A. J. J., & Lloyd-Jones, G. C. (2013). Selection of boron reagents for Suzuki–Miyaura coupling. Chemical Society Reviews, 43(1), 412–443. https://doi.org/10.1039/c3cs60197h
Zhong, Q., Ngim, K. K., Sun, M., Li, J., Deese, A., & Chetwyn, N. P. (2012). Strategies for the analysis of highly reactive pinacolboronate esters. Journal of Chromatography A, 1229, 216–222. https://doi.org/10.1016/j.chroma.2012.01.050
Achilli, C., et al. (2013). Susceptibility to hydrolysis of phenylboronic pinacol esters at physiological pH. Central European Journal of Chemistry, 11, 137–139.
Sandford, C., & Aggarwal, V. K. (2014). Selection of boron reagents for Suzuki–Miyaura coupling. Chemical Society Reviews, 43(1), 166–178.