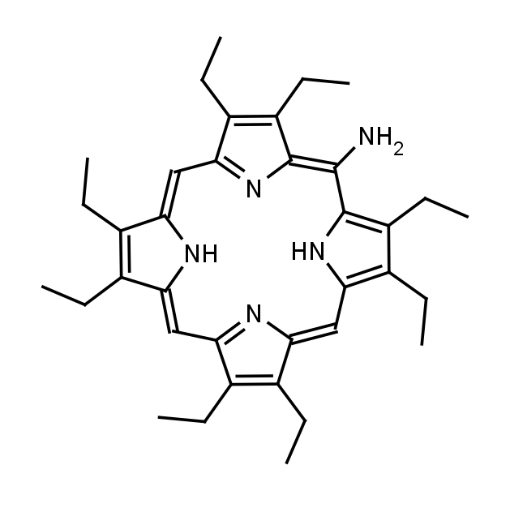
Name: 5-Amino Octaethylporphine
Molecular Formula: C36H47N5
CAS#: 3134-01-8
SMILES: CCC1=C(/C=C2C(CC)=C(CC)C3=N/2)NC(/C=C4N=C(/C=C(C(CC)=C/5CC)\NC5=C3\N)C(CC)=C\4CC)=C1CC
MDL#:
Catalog#: O34861
Molecular weight: 549.79 g/mol
Other names:
- 5-(amino)-2,3,7,8,12,13,17,18-(octaethyl)porphyrinName 2
- Amino-OEP
- 5-Amino-OEP
Fields of Interest: materials science, photodynamic therapy (PDT), sensor technology, catalysis
Background & Applications: Amino-octaethylporphyrin (Amino-OEP) is a stable, sterically hindered porphyrin derivative featuring an amino (-NH₂) substituent and ethyl groups at the β-positions of its pyrrole rings. This configuration enhances its solubility, chemical versatility, and electronic properties. The amino group facilitates covalent attachment to other molecules or materials, making Amino-OEP valuable in catalysis, photodynamic therapy, sensors, and materials science. It exhibits characteristic porphyrin absorption spectra, with a strong Soret band and Q-bands, which are useful in optical and spectroscopic applications. Amino-OEP is synthesized through condensation reactions, typically followed by post-functionalization to introduce the amino group. Its unique combination of structural stability and functional flexibility underpins its wide-ranging applications in advanced chemical and biomedical fields.
Appearance: Purple Solid
Purity: >95%
Storage: Stable at room temperature
Solubility: Soluble in organic solvents such as Chloroform, Acetone, Pyridine
Literature:
Smith, K. M., “Porphyrins and Metalloporphyrins: Synthesis and Applications,” Academic Press, 2011.
Kadish, K. M., Smith, K. M., & Guilard, R. “The Porphyrin Handbook,” Academic Press, 2000.