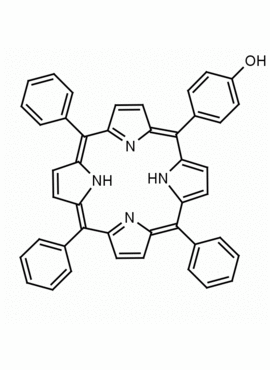
5-(4-hydroxyphenyl)-10,15,20-triphenyl porphine CAS: 87345-22-0 MDL: MFCD09038998
Molecular weight: 630.735 g/mol
Molecular Formula: C44H30N4O
CAS Number: 87345-22-0
Storage: Store at room temperature, protect from light.
Synonyms: 4-(10,15,20-Triphén
Fields of Interest: Synthetic Porphyrins, Mono Functionalized Porphyrins
Background: 5-(4-hydroxyphenyl)-10,15,20-triphenyl porphine is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals.
References:
1.) Cheng, et al. Red Light-Triggered Intracellular Carbon Monoxide Release Enables Selective Eradication of MRSA Infection. Angew. Chem. Int. Ed.Volume 60, Issue 24. June 7, 2021, Pages 13513-13520.https://doi.org/10.1002/anie.202104024
2.) Ruan, et al. The synthesis of star-shaped poly(N-isopropylacrylamide) with two zinc porphyrins as the core and end groups via ATRP and “CLICK” chemistry and a photocatalytic performance study. New J. Chem., 2020, 44, 2781-2787. https://doi.org/10.1039/C9NJ05802H
3.) Rojas-Montoya, et al. Synthesis and photophysical properties of novel pyrene–metalloporphyrin dendritic systems. Dalton Trans., 2019,48, 10435-10447. https://doi.org/10.1039/C9DT00855A
4.) D’Souza, et al. Energy Transfer Followed by Electron Transfer in a Supramolecular Triad Composed of Boron Dipyrrin, Zinc Porphyrin, and Fullerene: A Model for the Photosynthetic Antenna-Reaction Center Complex. J. Am. Chem. Soc. 2004, 126, 25, 7898–7907. https://doi.org/10.1021/ja030647u
5.) Ngen, et al. Evaluation of delocalized lipophilic cationic dyes as delivery vehicles for photosensitizers to mitochondria. Bioorganic & Medicinal Chemistry. Volume 17, Issue 18, 15 September 2009, Pages 6631-6640. https://doi.org/10.1016/j.bmc.2009.07.074
6.) Wang, et al. Novel phosphoramidates with porphine and nitrogenous drug: One-pot synthesis and orientation to cancer cells. European Journal of Medicinal Chemistry. Volume 45, Issue 3, March 2010, Pages 890-895.