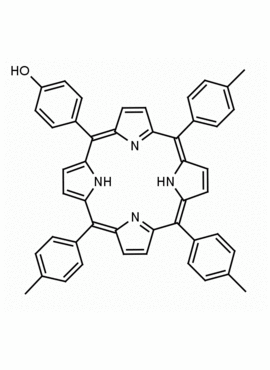
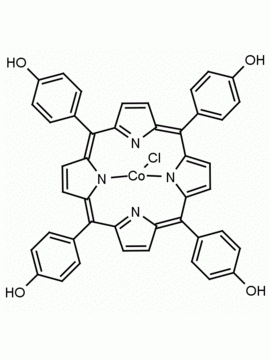
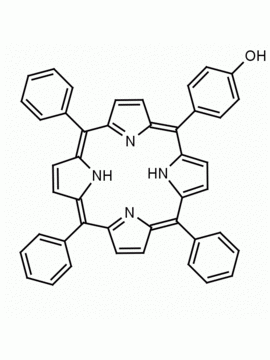
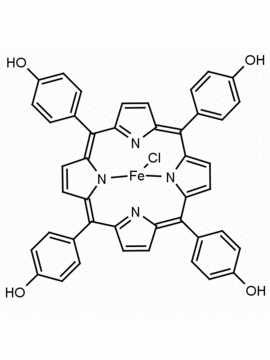
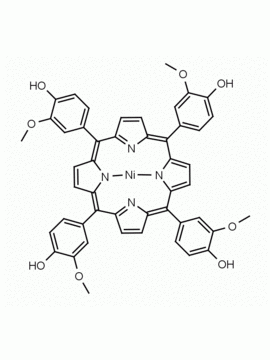
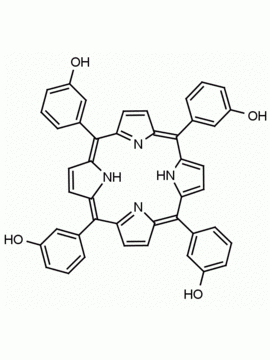
5-(4-hydroxyphenyl)-10,15,20-(4-methylphenyl) porphine 5-(4-hydroxyphenyl)-10,15,20-(4-tolyl) porphine CAS: 57412-08-5 MDL:
Molecular weight: 672.815 g/mol
Molecular Formula: C47H36N4O
CAS Number: 57412-08-5
Storage: Store at room temperature, protect from light.
Synonyms: 4-[10,15,20-Tris(4-
Fields of Interest: Synthetic Porphyrins, Supramolecular Chemistry, Artificial Photosynthesis
Background: 5-(4-hydroxyphenyl)-10,15,20-(4-methylphenyl) porphine is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals. 5-(4-hydroxyphenyl)-10,15,20-(4-methylphenyl) porphine was used for the construction of a supramolecular tetrad possessing the properties of multi-step energy and electron transfer is demonstrated.1 5-(4-hydroxyphenyl)-10,15,20-(4-methylphenyl) porphine was used for the construction of a diiridium (III) porphyrin which was used for the catalytic Hydrogenolysis of carbon–carbon σ-bonds using water.2 ATRP of monomers constructed from 5-(4-hydroxyphenyl)-10,15,20-(4-methylphenyl) porphine was used to create functional porphyrin polystyrene nano-architectures.3 5-(4-hydroxyphenyl)-10,15,20-(4-methylphenyl) porphine was used to construct diporphyrins ligands for the construction of Ce(IV) sandwich complexes.4 Zn(II) 5-(4-hydroxyphenyl)-10,15,20-(4-methylphenyl) porphine covalently linked to a phthalocyanine coordinated to a fullerene derivative exhibited ultrafast energy transfer and is a photosynthetic antenna-reaction center mimic.5 5-(4-hydroxyphenyl)-10,15,20-(4-methylphenyl) porphine was functionalized into a porphyrin azide derivative for click chemistry.6
References:
1.) Badgurjar, et al. One-Photon Excitation Followed by a Three-Step Sequential Energy–Energy–Electron Transfer Leading to a Charge-Separated State in a Supramolecular Tetrad Featuring Benzothiazole–Boron-Dipyrromethene–Zinc Porphyrin–C60. Chemistry, a European Journal. Volume 27, Issue 6, January 26, 2021, Pages 2184-2195. https://doi.org/10.1002/chem.202004262
2.) Fong, et al. Hydrogenolysis of carbon–carbon σ-bonds using water catalysed by semi-rigid diiridium(iii) porphyrins. New J. Chem., 2019,43, 3656-3659. https://doi.org/10.1039/C8NJ05664A
3.) Loos, et al. Construction of functional porphyrin polystyrene nano-architectures by ATRP. Chem. Commun., 2005, 60-62. https://doi.org/10.1039/B412067A
4.) Buchler, et al. Oxidation and Reduction of Cerium(IV) Sandwich Complexes with Porphyrin Ligands Linked by Aliphatic Diether Bridges of Variable Chain Length. EurJIC. Volume 1998, Issue 4, April 1998, Pages 445-449. https://doi.org/10.1002/(SICI)1099-0682(199804)1998:4<445::AID-EJIC445>3.0.CO;2-D
5.) Chandra, et al. Supramolecular Tetrad Featuring Covalently Linked Bis(porphyrin)–Phthalocyanine Coordinated to Fullerene: Construction and Photochemical Studies. Chemistry, a European Journal. Volume 20, Issue 25, June 16, 2014, Pages 7725-7735. https://doi.org/10.1002/chem.201402402
6.) Punidha, et al. First Triazole-Bridged Unsymmetrical Porphyrin Dyad via Click Chemistry. J. Org. Chem. 2008, 73, 1, 323–326. https://doi.org/10.1021/jo702018s