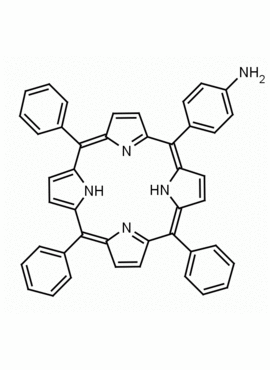
5-(4-aminophenyl)-10,15,20-triphenyl porphine CAS: 67605-64-5 MDL: MFCD01074434
Molecular weight: 629.8 g/mol
Molecular Formula: C44H31N5
CAS Number: 67605-64-5
Storage: Store at room temperature, protect from light.
Purity: >95%
Synonyms: 5-(4-aminophenyl)-10,15,20-triphenyl porphine, SCHEMBL3999312, CHEMBL3408510, C-0168, 5-(4-aminophenyl)-10,15,20-triphenylporphyrin, 5-(4-aminophenyl)-10,15,20-(triphenyl)porphyrin, 5-(4-aminophenyl)-10-15-20-(triphenyl)porphyrin, 5-(4-Aminophenyl)-10,15,20-tris(phenyl)porphyrin, 4-(5,10,20-Triphenyl-21H,23H-porphyrin-15-yl)aniline, 4-(10,15,20-Triphén
Fields of Interest: Nanocomposites, Catalysis, Photocatalysis, Metal Organic Frameworks, Immunoassay, Porphyrin Functionalized Materials
Background: 5-(4-aminophenyl)-10,15,20-triphenyl porphine is a synthetic porphyrin specialty chemical manufactured by Frontier Specialty Chemicals. 5-(4-aminophenyl)-10,15,20-triphenyl porphine was used to covalently functionalize graphene oxide.1 5-(4-aminophenyl)-10,15,20-triphenyl porphine was found to effectively chelate the radioisotope 64Cu and found to have favorable bio distribution in mice.2 Electropolymerization of 5-(4-aminophenyl)-10,15,20-triphenyl porphine and its Cu(II) adducts resulted in the precipitation of electro-conductive films.3 5-(4-aminophenyl)-10,15,20-triphenyl porphine was used to construct porphyrin containing light-responsive capsules for controlled drug release.4 5-(4-aminophenyl)-10,15,20-triphenyl porphine was used to synthesize an electrochemiluminescent porphyrin by coupling to a tetraphenylethylene derivative.5 5-(4-aminophenyl)-10,15,20-triphenyl porphine was used to synthesize porphyrin modified siloxanes.6
References:
1.) Karousis, et al. Graphene oxide with covalently linked porphyrin antennae: Synthesis, characterization and photophysical properties. J. Mater. Chem., 2011, 21, 109-117. 10.1039/C0JM00991A
2.) Aguilar-Ortiz, et al. Synthesis, characterization and evaluation of a Cu-labeled macrocyclic-porphyrin as a potential chelator for 64Cu-based radiopharmaceuticals. Journal of Radioanalytical and Nuclear Chemistry volume 320, pages 79–86 (2019). https://doi.org/10.1007/s10967-019-06454-4
3.) Tesakova, et al. Electroconductive films based on amino-substituted tetraphenylporphyrins and their metal copper complexes. Journal of Porphyrins and Phthalocyanines. Vol. 20, No. 07, pp. 793-803 (2016). https://doi.org/10.1142/S1088424616500930
4.) Li, et al. Porphyrin containing light-responsive capsules for controlled drug release. J. Mater. Chem., 2012,22, 4623-4626. https://doi.org/10.1039/C2JM16702F
5.) Zhang, et al. Switching the Photoluminescence and Electrochemiluminescence of Liposoluble Porphyrin in Aqueous Phase by Molecular Regulation. Angew. Chemie. Int. Ed. Volume 59, Issue51 December 14, 2020 Pages 23261-23267. https://doi.org/10.1002/anie.202010216
6.) Almeida, et al. (Aminophenyl)porphyrins as precursors for the synthesis of porphyrin-modified siloxanes. Journal of Porphyrins and Phthalocyanines Vol. 23, No. 09, pp. 1001-1012 (2019). https://doi.org/10.1142/S1088424619500573
7.) Labels for fluorescence immunoassays and intermediates for their preparation, Schmidt, Dieter Steffen, Hans, Eur. Pat. Appl. (1984), EP 127797 A1 19841212,
8.) Syntheses and characterization of molybdenum/zinc porphyrin dimers bridged by aromatic linkers, Song, Sai-Nan Li, Dong-Mei Wu, Jun-Feng Zhuang, Chang-Fu Ding, Hong Song, Wen-Bo Cui, Li-Feng Cao, Geng-Zhen Liu, Guo-Fa, European Journal of Inorganic Chemistry (2007), (13), 1844-1853. DOI:10.1002/ejic.200600853