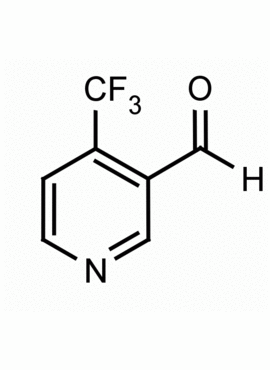
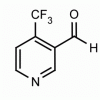
4-(Trifluoromethyl)-3-formylpyridine CAS: 1083197-78-7 MDL: MFCD07781251
Molecular weight: 175.11 g/mol
Molecular Formula: C7H4F3NO
CAS Number: 1083197-78-7
Storage: Store at 2-8 Co, under dry conditions.
Synonyms: 4-(Trifluoromethyl)nicotinaldehyde,083197-78-7, 4-TRIFLUOROMETHYL-3-FORMYLPYRIDINE, 4-(trifluoromethyl)pyridine-3-carbaldehyde, 872626-76-1, 4-(Trifluoromethyl)-3-formylpyridine, 4-Trifluoromethyl-pyridine-3-carbaldehyde, 4-(trifluoromethyl)-3-pyridinecarboxaldehyde, PubChem5155, 3-Pyridinecarboxaldehyde, 4-(trifluoromethyl)-, SCHEMBL844273
Uses: Synthesis building block, Organic Synthesis, pyridine nitrogen heterocycle, synthesis, aldehyde reactions, organofluorine
4-(Trifluoromethyl)-3-formylpyridine, is a synthetic fine chemical useful in the synthesis of pharmaceuticals and fine organic chemicals.
Selected References:
- Bayrak, H.; Demirbas, A.; Karaoglu, S. A.; Demirbas, N., Synthesis of some new 1,2,4-triazoles, their Mannich and Schiff bases and evaluation of their antimicrobial activities. Eur. J. Med. Chem. 2009, 44 (3), 1057-1066.
- Chang, J.; Zhao, K.; Pan, S., Synthesis of 2-arylbenzoxazoles via DDQ promoted oxidative cyclization of phenolic Schiff bases-a solution-phase strategy for library synthesis. Tetrahedron Lett. 2002, 43 (6), 951-954.
- Evdokimov, N. M.; Magedov, I. V.; Kireev, A. S.; Kornienko, A., One-Step, Three-Component Synthesis of Pyridines and 1,4-Dihydropyridines with Manifold Medicinal Utility. Org. Lett. 2006, 8 (5), 899-902.
- Johnson, M. P.; Baez, M.; Jagdmann, G. E., Jr.; Britton, T. C.; Large, T. H.; Callagaro, D. O.; Tizzano, J. P.; Monn, J. A.; Schoepp, D. D., Discovery of Allosteric Potentiators for the Metabotropic Glutamate 2 Receptor: Synthesis and Subtype Selectivity of N-(4-(2-Methoxyphenoxy)phenyl)-N-(2,2,2- trifluoroethylsulfonyl)pyrid-3-ylmethylamine. J. Med. Chem. 2003, 46 (15), 3189-3192.
- Kallander, L. S.; Lu, Q.; Chen, W.; Tomaszek, T.; Yang, G.; Tew, D.; Meek, T. D.; Hofmann, G. A.; Schulz-Pritchard, C. K.; Smith, W. W.; Janson, C. A.; Ryan, M. D.; Zhang, G.-F.; Johanson, K. O.; Kirkpatrick, R. B.; Ho, T. F.; Fisher, P. W.; Mattern, M. R.; Johnson, R. K.; Hansbury, M. J.; Winkler, J. D.; Ward, K. W.; Veber, D. F.; Thompson, S. K., 4-Aryl-1,2,3-triazole: A Novel Template for a Reversible Methionine Aminopeptidase 2 Inhibitor, Optimized To Inhibit Angiogenesis in Vivo. J. Med. Chem. 2005, 48 (18), 5644-5647.
- Kemnitzer, W.; Drewe, J.; Jiang, S.; Zhang, H.; Wang, Y.; Zhao, J.; Jia, S.; Herich, J.; Labreque, D.; Storer, R.; Meerovitch, K.; Bouffard, D.; Rej, R.; Denis, R.; Blais, C.; Lamothe, S.; Attardo, G.; Gourdeau, H.; Tseng, B.; Kasibhatla, S.; Cai, S. X., Discovery of 4-Aryl-4H-chromenes as a New Series of Apoptosis Inducers Using a Cell- and Caspase-based High-Throughput Screening Assay. 1. Structure-Activity Relationships of the 4-Aryl Group. J. Med. Chem. 2004, 47 (25), 6299-6310.
- Kemnitzer, W.; Drewe, J.; Jiang, S.; Zhang, H.; Zhao, J.; Crogan-Grundy, C.; Xu, L.; Lamothe, S.; Gourdeau, H.; Denis, R.; Tseng, B.; Kasibhatla, S.; Cai, S. X., Discovery of 4-Aryl-4H-chromenes as a New Series of Apoptosis Inducers Using a Cell- and Caspase-Based High-Throughput Screening Assay. 3. Structure-Activity Relationships of Fused Rings at the 7,8-Positions. J. Med. Chem. 2007, 50 (12), 2858-2864.
- Kemnitzer, W.; Kasibhatla, S.; Jiang, S.; Zhang, H.; Zhao, J.; Jia, S.; Xu, L.; Crogan-Grundy, C.; Denis, R.; Barriault, N.; Vaillancourt, L.; Charron, S.; Dodd, J.; Attardo, G.; Labrecque, D.; Lamothe, S.; Gourdeau, H.; Tseng, B.; Drewe, J.; Cai, S. X., Discovery of 4-aryl-4H-chromenes as a new series of apoptosis inducers using a cell- and caspase-based high-throughput screening assay. 2. Structure-activity relationships of the 7- and 5-, 6-, 8-positions. Bioorg. Med. Chem. Lett. 2005, 15 (21), 4745-4751.
- Vasuki, G.; Kumaravel, K., Rapid four-component reactions in water: synthesis of pyranopyrazoles. Tetrahedron Lett. 2008, 49 (39), 5636-5638.
- Zarrinmayeh, H.; Nunes, A. M.; Ornstein, P. L.; Zimmerman, D. M.; Arnold, M. B.; Schober, D. A.; Gackenheimer, S. L.; Bruns, R. F.; Hipskind, P. A.; Britton, T. C.; Cantrell, B. E.; Gehlert, D. R., Synthesis and Evaluation of a Series of Novel 2-[(4-Chlorophenoxy)methyl]benzimidazoles as Selective Neuropeptide Y Y1 Receptor Antagonists. J. Med. Chem. 1998, 41 (15), 2709-2719.