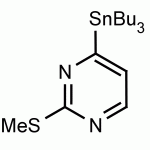
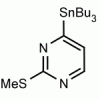
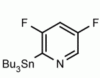
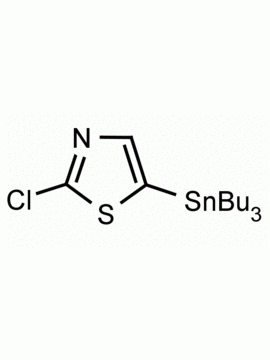
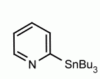
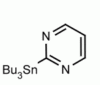
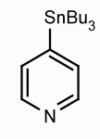
4-(Tributylstannyl)-2-thiomethylpyrimidine Pyrimidine, 2-(methylthio)-4-(tributylstannyl)- CAS: 123061-49-4 MDL: MFCD01319026
Molecular weight: 415.224 g/mol
Molecular Formula: C17H32N2SSn
CAS Number: 123061-49-4
Storage: Store at room temperature, protect from light
Synonyms: 2-(Methylthio)-4-(tributylstan
Field of Interest: Stille Cross Coupling Reaction
Selected References:
1.) Allred, Gary D., and Lanny S. Liebeskind. Copper-Mediated Cross-Coupling of Organostannanes with Organic Iodides at or below Room Temperature. Journal of the American Chemical Society 118, (1996): 2748–49. https://doi.org/10.1021/JA9541239.
2.) Dubbaka, Srinivas Reddy, and Pierre. Vogel. Palladium-Catalyzed Stille Cross-Couplings of Sulfonyl Chlorides and Organostannanes. Journal of the American Chemical Society125, (2003): 15292–93. https://doi.org/10.1021/ja038328q.
3.) Echavarren, Antonio M., and J. K. Stille. Palladium-Catalyzed Carbonylative Coupling of Aryl Triflates with Organostannanes. Journal of the American Chemical Society 110, (1988): 1557–65. https://doi.org/10.1021/ja00213a032.
4.) Palladium-Catalyzed Coupling of Aryl Triflates with Organostannanes. Journal of the American Chemical Society 109, (1987): 5478–86. https://doi.org/10.1021/ja00252a029.
5.) Egi, Masahiro, and Lanny S. Liebeskind. Heteroaromatic Thioether-Organostannane Cross-Coupling. Organic Letters 5, (2003): 801–2. https://doi.org/10.1021/ol0273497.
6.)Espinet, Pablo, and Antonio M. Echavarren. C-C Coupling: The Mechanisms of the Stille Reaction. Angewandte Chemie, International Edition 43, (2004): 4704–34. https://doi.org/10.1002/anie.200300638.
7.) Farina, Vittorio. New Perspectives in the Cross-Coupling Reactions of Organostannanes. Pure and Applied Chemistry 68, (1996): 73–78. https://doi.org/10.1351/pac199668010073.
8.) Finet, Jean-Pierre, Alexey Yu. Fedorov, Sebastien Combes, and Gerard. Boyer. Recent Advances in Ullmann Reaction: Copper(II) Diacetate Catalyzed N-, O- and S-Arylation Involving Polycoordinate Heteroatomic Derivatives. Current Organic Chemistry 6, (2002): 597–626. https://doi.org/10.2174/1385272023374058.
9.) Garcia-Martinez, Joaquin C., Raphael Lezutekong, and Richard M. Crooks. Dendrimer-Encapsulated Pd Nanoparticles as Aqueous, Room-Temperature Catalysts for the Stille Reaction. Journal of the American Chemical Society 127, (2005): 5097–5103. https://doi.org/10.1021/ja042479r.
10.) Grasa, Gabriela A., and Steven P. Nolan. Palladium/Imidazolium Salt Catalyzed Coupling of Aryl Halides with Hypervalent Organostannates. Organic Letters 3, (2001): 119–22. https://doi.org/10.1021/ol006827f.
11.) Hayashi, Tamio, and Mitsuo. Ishigedani. Rhodium-Catalyzed Asymmetric Arylation of Imines with Organostannanes. Asymmetric Synthesis of Diarylmethylamines. Journal of the American Chemical Society 122, (2000): 976–77. https://doi.org/10.1021/ja9927220.
12.) Kang, Suk-Ku, Tokutaro Yamaguchi, Tae-Hyun Kim, and Pil-Su. Ho. Copper-Catalyzed Cross-Coupling and Carbonylative Cross-Coupling of Organostannanes and Organoboranes with Hypervalent Iodine Compounds. Journal of Organic Chemistry 61, (1996): 9082–83. https://doi.org/10.1021/JO962033W.
13.) Labadie, Jeff W., and J. K. Stille. Mechanisms of the Palladium-Catalyzed Couplings of Acid Chlorides with Organotin Reagents. Journal of the American Chemical Society 105, (1983): 6129–37. https://doi.org/10.1021/ja00357a026.
14.) Liu, Songbai, Ying Yu, and Lanny S. Liebeskind. N-Substituted Imines by the Copper-Catalyzed N-Imination of Boronic Acids and Organostannanes with O-Acyl Ketoximes. Organic Letters 9, (2007): 1947–50. https://doi.org/10.1021/ol070561w.
15.) Navarre, Laure, Remi Martinez, Jean-Pierre Genet, and Sylvain. Darses. Access to Enantioenriched α-Amino Esters via Rhodium-Catalyzed 1,4-Addition/Enantioselective Protonation. Journal of the American Chemical Society 130, (2008): 6159–69. https://doi.org/10.1021/ja710691p.
16.) Scott, William J., and John E. McMurry. Olefin Synthesis via Organometallic Coupling Reactions of Enol Triflates. Accounts of Chemical Research 21, (1988): 47–54. https://doi.org/10.1021/ar00146a001.
17.) Scott, William J., and J. K. Stille. Palladium-Catalyzed Coupling of Vinyl Triflates with Organostannanes. Synthetic and Mechanistic Studies. Journal of the American Chemical Society 108, (1986): 3033–40. https://doi.org/10.1021/ja00271a037.
18.) Shen, Wang, and Le. Wang. The Stille Reaction of 1,1-Dibromo-1-Alkenes: Preparation of Trisubstituted Alkenes and Internal Alkynes. Journal of Organic Chemistry 64, (1999): 8873–79. https://doi.org/10.1021/jo991116k.
19.) Urkalan, Kaveri Balan, and Matthew S. Sigman. Palladium-Catalyzed Oxidative Intermolecular Difunctionalization of Terminal Alkenes with Organostannanes and Molecular Oxygen. Angewandte Chemie, International Edition 48, (2009): 3146–49, S3146/1-S3146/64. https://doi.org/10.1002/anie.200900218.
20.) Wittenberg, Ruediger, Jiri Srogl, Masahiro Egi, and Lanny S. Liebeskind. Ketone Synthesis under Neutral Conditions. Cu(I) Diphenylphosphinate-Mediated, Palladium-Catalyzed Coupling of Thiol Esters and Organostannanes. Organic Letters 5, (2003): 3033–35. https://doi.org/10.1021/ol034962x.