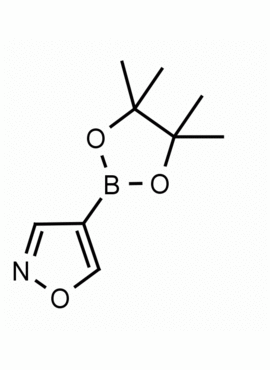
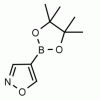
4-Isoxazoleboronic acid pinacol ester CAS: 928664-98-6 MDL: MFCD06657891
Molecular weight: 195.03 g/mol
Molecular Formula: C9H14BNO3
CAS Number: 928664-98-6
Storage: Store at 2-8 Co, under dry conditions.
Synonyms: 4-Isoxazoleboronic acid pinacol ester, 4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)isoxazole, ISOXAZOLE-4-BORONIC ACID PINACOL ESTER
MFCD06657891, 4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-1,2-oxazole, 4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-isoxazole, Isoxazole, 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-, SCHEMBL142345
Uses: Synthesis building block, Organic Synthesis, Transition Metal Coupling
4-Isoxazoleboronic acid pinacol ester, is a synthetic specialty chemical useful in the synthesis of pharmaceuticals and specialty organic chemicals.
Isoxazoles in Medicine:
1.) Zimecki M, Bąchor U, Mączyński M. Isoxazole Derivatives as Regulators of Immune Functions. Molecules. 2018;23(10):2724. Published 2018 Oct 22. doi:10.3390/molecules23102724
2.) Zhu J, Mo J, Lin HZ, Chen Y, Sun HP. The recent progress of isoxazole in medicinal chemistry. Bioorg Med Chem. 2018 Jul 23;26(12):3065-3075. doi: 10.1016/j.bmc.2018.05.013. Epub 2018 May 28. PMID: 29853341.
Pinacol Ester Derivative Coupling Reactions
1.) Palladium(0)-Catalyzed Cross-Cross-Coupling Reaction of Alkoxydiboron with Haloarenes: A Direct Procedure for Arylboronic Esters, Ishiyama, Tatsuo, Murata, Miki, Miyaura, Norio, Journal of Organic Chemistry (1995), 60(23), 7508-10. DOI:10.1021/jo00128a024
2.) Cross Coupling Reactions of Chiral Secondary Organoboronic Esters With Retention of Configuration, Imao, Daisuke, Glasspoole, Ben W., Laberge, Veronique S., Crudden, Cathleen M., Journal of the American Chemical Society (2009), 131(14), 5024-5025. DOI:10.1021/ja8094075
3.) Palladium-Catalyzed Cross-Coupling Reaction of Bis(pinacolato)diboron with 1-Alkenyl Halides or Triflates: Convenient Synthesis of Unsymmetrical 1,3-Dienes via the Borylation-Coupling Sequence, Takagi, Jun, Takahashi, Kou, Ishiyama, Tatsuo, Miyaura, Norio, Journal of the American Chemical Society (2002), 124(27), 8001-8006. DOI:10.1021/ja0202255
4.) Functionalized olefin cross-coupling to construct carbon-carbon bonds, Lo, Julian C., Gui, Jinghan, Yabe, Yuki, Pan, Chung-Mao, Baran, Phil S., Nature (London, United Kingdom) (2014), 516(7531), 343-348. DOI:10.1038/nature14006.
5.) The Synthesis of Highly Substituted Isoxazoles by Electrophilic Cyclization: An Efficient Synthesis of Valdecoxib, Waldo, Jesse P., Larock, Richard C., Journal of Organic Chemistry (2007), 72(25), 9643-9647. DOI:10.1021/jo701942e.
6.) Arenes to anilines and aryl ethers by sequential iridium-catalyzed borylation and copper-catalyzed coupling, Tzschucke, C. Christoph, Murphy, Jaclyn M., Hartwig, John F., Organic Letters (2007), 9(5), 761-764. DOI:10.1021/ol062902w
7.) A synthesis of allyboronates via the palladium(0)-catalyzed cross-coupling reaction of bis(pinacolato)diboron with allylic acetates, Ishiyama, Tatsuo, Ahiko, Taka-aki, Miyaura, Norio, Tetrahedron Letters (1996), 37(38), 6889-6892. DOI:10.1016/0040-4039(96)01505-5
8.) Rapid synthesis of 3-amino-imidazopyridines by a microwave-assisted four-component coupling in one pot, DiMauro, Erin F., Kennedy, Joseph M., Journal of Organic Chemistry (2007), 72(3), 1013-1016, DOI:10.1021/jo0622072
9.) Iron-Catalyzed C(sp2)-H Bond Functionalization with Organoboron Compounds, Shang, Rui, Ilies, Laurean, Asako, Sobi, Nakamura, Eiichi, Journal of the American Chemical Society (2014), 136(41), 14349-14352. DOI:10.1021/ja5070763
10.) Copper-Promoted Coupling of Vinyl Boronates and Alcohols: A Mild Synthesis of Allyl Vinyl Ethers, Shade, Ryan E., Hyde, Alan M., Olsen, John-Carl, Merlic, Craig A., Journal of the American Chemical Society (2010), 132(4), 1202-1203. DOI:10.1021/ja907982w