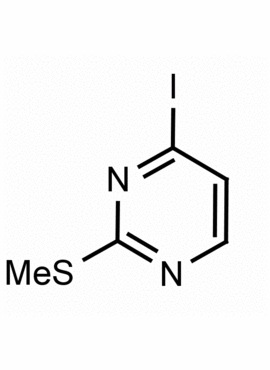
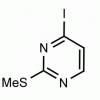
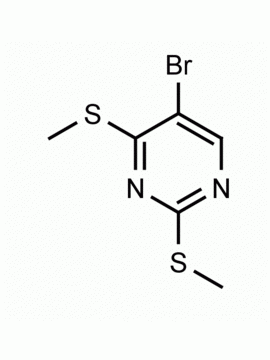
4-Iodo-2-(methylthio)pyrimidine CAS: 1122-74-3 MDL: MFCD01319020
Molecular weight: 252.08 g/mol
Molecular Formula: C5H5IN2S
CAS Number: 1122-74-3
Storage: Store at 2-8 Co, under dry conditions.
Synonyms: 4-Iodo-2-(methylthio)pyrimidine, 1122-74-3, 4-iodo-2-methylsulfanylpyrimidine, 4-iodo-2-(methylsulfanyl)pyrimidine, 4-lodo-2-(methylthio)pyrimidine, 4-iodo-2-methylsulfanyl-pyrimidine, 4-Iodo-2-methylthiopyrimidine, Pyrimidine, 4-iodo-2-(methylthio)-, 4-IODO-2-(METHYLTHIO)-PYRIMIDINE, 4-Iodo-2-(methylsulphanyl)pyrimidine, MFCD01319020, PubChem5294, 2-methylthio4-iodopyrimidine, 2-methylthio-4-iodopyrimidine, SCHEMBL164463
Uses: Synthesis building block, Organic Synthesis, pyrimidine nitrogen heterocycle, synthesis, iodo arene reactions
4-Iodo-2-(methylthio)pyrimidine, is a synthetic fine chemical useful in the synthesis of pharmaceuticals and fine organic chemicals.
Iodoarene Reactions and Selected References:
1.) Catellani, Marta, Franco Frignani, and Armando. Rangoni. A Complex Catalytic Cycle Leading to a Regioselective Synthesis of o,o’-Disubstituted Vinylarenes. Angewandte Chemie, International Edition in English 36,(1997): 119–122. https://doi.org/10.1002/anie.199701191.
2.) Chalker, Justin M., Charlotte S. C. Wood, and Benjamin G. Davis. A Convenient Catalyst for Aqueous and Protein Suzuki-Miyaura Cross-Coupling. Journal of the American Chemical Society 131, (2009): 16346–47. https://doi.org/10.1021/ja907150m.
3.) Clark, James H., Duncan J. Macquarrie, and Egid B. Mubofu. Preparation of a Novel Silica-Supported Palladium Catalyst and Its Use in the Heck Reaction. Green Chemistry 2, (2000): 53–56. https://doi.org/10.1039/a908685d.
4.) Davies, Ian W., Louis Matty, David L. Hughes, and Paul J. Reider. Are Heterogeneous Catalysts Precursors to Homogeneous Catalysts? Journal of the American Chemical Society 123, (2001): 10139–40. https://doi.org/10.1021/ja016877v.
5.) Dohi, Toshifumi, Akinobu Maruyama, Yutaka Minamitsuji, Naoko Takenaga, and Yasuyuki. Kita. First Hypervalent Iodine(III)-Catalyzed C-N Bond Forming Reaction: Catalytic Spirocyclization of Amides to N-Fused Spirolactams. Chemical Communications (Cambridge, United Kingdom), (2007): 1224–26. https://doi.org/10.1039/B616510A.
6.) Fernandez-Rodriguez, Manuel A., and John F. Hartwig. A General, Efficient, and Functional-Group-Tolerant Catalyst System for the Palladium-Catalyzed Thioetherification of Aryl Bromides and Iodides. Journal of Organic Chemistry 74, (2009): 1663–72. https://doi.org/10.1021/jo802594d.
7.) Grushin, Vladimir V., and Howard. Alper. Alkali-Induced Disproportionation of Palladium(II) Tertiary Phosphine Complexes, [L2PdCl2], to LO and Palladium(O). Key Intermediates in the Biphasic Carbonylation of ArX Catalyzed by [L2PdCl2]. Organometallics 12, (1993): 1890–1901. https://doi.org/10.1021/om00029a052.
8.) Ishiyama, Tatsuo, Hiroe Kizaki, Norio Miyaura, and Akira. Suzuki. Synthesis of Unsymmetrical Biaryl Ketones via Palladium-Catalyzed Carbonylative Cross-Coupling Reaction of Arylboronic Acids with Iodoarenes. Tetrahedron Letters 34, (1993): 7595–98. https://doi.org/10.1016/S0040-4039(00)60409-4.
9.) Join, Benoit, Takuya Yamamoto, and Kenichiro. Itami. Iridium Catalysis for C-H Bond Arylation of Heteroarenes with Iodoarenes. Angewandte Chemie, International Edition 48, (2009): 3644–47, S3644/1-S3644/79. https://doi.org/10.1002/anie.200806358.
10.) Lebrasseur, Nathalie, and Igor. Larrosa. Room Temperature and Phosphine Free Palladium Catalyzed Direct C-2 Arylation of Indoles. Journal of the American Chemical Society 130, (2008): 2926–27. https://doi.org/10.1021/ja710731a.
11.) Li, Yin, Xiaoyong M. Hong, David M. Collard, and Mostafa A. El-Sayed. Suzuki Cross-Coupling Reactions Catalyzed by Palladium Nanoparticles in Aqueous Solution. Organic Letters 2, (2000): 2385–88. https://doi.org/10.1021/ol0061687.
12.) Morimoto, Hiroyuki, Tetsu Tsubogo, Nichole D. Litvinas, and John F. Hartwig. A Broadly Applicable Copper Reagent for Trifluoromethylations and Perfluoroalkylations of Aryl Iodides and Bromides. Angewandte Chemie, International Edition 50, (2011): 3793–98, S3793/1-S3793/78. https://doi.org/10.1002/anie.201100633.
13.) Oishi, Masahiro, Hideaki Kondo, and Hideki. Amii. Aromatic Trifluoromethylation Catalytic in Copper. Chemical Communications (Cambridge, United Kingdom), (2009): 1909–11. https://doi.org/10.1039/b823249k.
14.) Quideau, Stephane, Gildas Lyvinec, Melanie Marguerit, Katell Bathany, Aurelie Ozanne-Beaudenon, Thierry Buffeteau, Dominique Cavagnat, and Alain. Chenede. Asymmetric Hydroxylative Phenol Dearomatization through In Situ Generation of Iodanes from Chiral Iodoarenes and M-CPBA. Angewandte Chemie, International Edition 48, (2009): 4605–9, S4605/1-S4605/104. https://doi.org/10.1002/anie.200901039.
15.) Rodriguez, Nuria, Jose A. Romero-Revilla, M. Angeles Fernandez-Ibanez, and Juan C. Carretero. Palladium-Catalyzed N-(2-Pyridyl)Sulfonyl-Directed C(Sp3)-H γ-Arylation of Amino Acid Derivatives. Chemical Science 4, (2013): 175–79. https://doi.org/10.1039/C2SC21162A.
16.) Tye, Jesse W., Zhiqiang Weng, Adam M. Johns, Christopher D. Incarvito, and John F. Hartwig. Copper Complexes of Anionic Nitrogen Ligands in the Amidation and Imidation of Aryl Halides. Journal of the American Chemical Society 130, (2008): 9971–83. https://doi.org/10.1021/ja076668w.
17.) Uyanik, Muhammet, Takeshi Yasui, and Kazuaki. Ishihara. Chiral Hypervalent Iodine-Catalyzed Enantioselective Oxidative Kita Spirolactonization of 1-Naphthol Derivatives and One-Pot Diastereo-Selective Oxidation to Epoxyspirolactones. Tetrahedron 66, (2010): 5841–51. https://doi.org/10.1016/j.tet.2010.04.060.
18.) Enantioselective Kita Oxidative Spirolactonization Catalyzed by In Situ Generated Chiral Hypervalent Iodine(III) Species. Angewandte Chemie, International Edition 49, (2010): 2175–77, S2175/1-S2175/79. https://doi.org/10.1002/anie.200907352.
19.) Wang, Guan-Wu, Ting-Ting Yuan, and Dan-Dan. Li. One-Pot Formation of C-C and C-N Bonds through Palladium-Catalyzed Dual C-H Activation: Synthesis of Phenanthridinones. Angewandte Chemie, International Edition 50, (2011): 1380–83, S1380/1-S1380/61. https://doi.org/10.1002/anie.201005874.
20.) Wang, Xiao-Chen, Wei Gong, Li-Zhen Fang, Ru-Yi Zhu, Suhua Li, Keary M. Engle, and Jin-Quan. Yu. Ligand-Enabled Meta-C-H Activation Using a Transient Mediator. Nature (London, United Kingdom) 519, (2015): 334–38. https://doi.org/10.1038/nature14214.