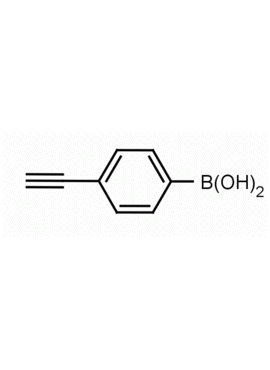
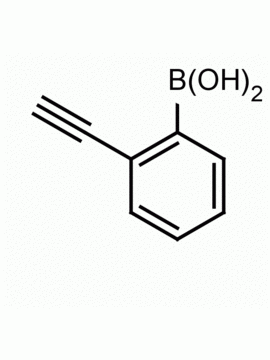
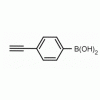4-Ethynylphenylboronic acid CAS: 263368-72-5 MDL: MFCD08689553
Molecular weight: 145.95 g/mol
Molecular Formula: C8H7BO2
CAS Number: 263368-72-5
Storage: Store at 2-8 Co, under dry conditions.
Synonyms: (4-Ethynylphenyl)boronic acid, 4-ETHYNYLPHENYLBORONIC ACID, 4-(DIHYDROXYBOROPHENYL)ACETYLENE, Boronic acid, B-(4-ethynylphenyl)-, B-(4-Ethynylphenyl)-boronic acid, Boronic acid, (4-ethynylphenyl)- (9CI), 4-ethynylbenzeneboronic acid
Uses: Synthesis building block, Organic Synthesis, alkyne, organoboron reactions
4-Ethynylphenylboronic acid, is a synthetic specialty chemical useful in the synthesis of pharmaceuticals and specialty organic chemicals.
Boronic Acid Derivative Coupling Reactions
1.) Altenhoff, Gereon, Richard Goddard, Christian W. Lehmann, and Frank. Glorius. Sterically Demanding, Bioxazoline-Derived N-Heterocyclic Carbene Ligands with Restricted Flexibility for Catalysis. Journal of the American Chemical Society 126, no. (2004): 15195–201. https://doi.org/10.1021/ja045349r.
2.) Arvela, Riina K., Nicholas E. Leadbeater, Michael S. Sangi, Victoria A. Williams, Patricia Granados, and Robert D. Singer. A Reassessment of the Transition-Metal Free Suzuki-Type Coupling Methodology. Journal of Organic Chemistry 70, no. (2005): 161–68. https://doi.org/10.1021/jo048531j.
3.) Billingsley, Kelvin, and Stephen L. Buchwald. Highly Efficient Monophosphine-Based Catalyst for the Palladium-Catalyzed Suzuki-Miyaura Reaction of Heteroaryl Halides and Heteroaryl Boronic Acids and Esters. Journal of the American Chemical Society 129, no. (2007): 3358–66. https://doi.org/10.1021/ja068577p.
4.) Billingsley, Kelvin L., Kevin W. Anderson, and Stephen L. Buchwald. A Highly Active Catalyst for Suzuki-Miyaura Cross-Coupling Reactions of Heteroaryl Compounds. Angewandte Chemie, International Edition 45, no. (2006): 3484–88. https://doi.org/10.1002/anie.200600493.
5.) Gillis, Eric P., and Martin D. Burke. A Simple and Modular Strategy for Small Molecule Synthesis: Iterative Suzuki-Miyaura Coupling of B-Protected Haloboronic Acid Building Blocks. Journal of the American Chemical Society 129, no. (2007): 6716–17. https://doi.org/10.1021/ja0716204.
6.) Herradura, Prudencio S., Kathleen A. Pendola, and R. Kiplin. Guy. Copper-Mediated Cross-Coupling of Aryl Boronic Acids and Alkyl Thiols. Organic Letters 2, no. (2000): 2019–22. https://doi.org/10.1021/ol005832g.
7.) Kataoka, Noriyasu, Quinetta Shelby, James P. Stambuli, and John F. Hartwig. Air Stable, Sterically Hindered Ferrocenyl Dialkylphosphines for Palladium-Catalyzed C-C, C-N, and C-O Bond-Forming Cross-Couplings. Journal of Organic Chemistry 67, no. (2002): 5553–66. https://doi.org/10.1021/jo025732j.
8.) Kinzel, Tom, Yong Zhang, and Stephen L. Buchwald. A New Palladium Precatalyst Allows for the Fast Suzuki-Miyaura Coupling Reactions of Unstable Polyfluorophenyl and 2-Heteroaryl Boronic Acids. Journal of the American Chemical Society 132, no. (2010): 14073–75. https://doi.org/10.1021/ja1073799.
9.) Knapp, David M., Eric P. Gillis, and Martin D. Burke. A General Solution for Unstable Boronic Acids: Slow-Release Cross-Coupling from Air-Stable MIDA Boronates. Journal of the American Chemical Society 131, no. (2009): 6961–63. https://doi.org/10.1021/ja901416p.
10.) Liebeskind, Lanny S., and Jiri. Srogl. Thiol Ester-Boronic Acid Coupling. A Mechanistically Unprecedented and General Ketone Synthesis. Journal of the American Chemical Society 122, no. (2000): 11260–61. https://doi.org/10.1021/ja005613q.
11.) McGuinness, David S., and Kingsley J. Cavell. Donor-Functionalized Heterocyclic Carbene Complexes of Palladium(II): Efficient Catalysts for C-C Coupling Reactions. Organometallics 19, no. (2000): 741–48. https://doi.org/10.1021/om990776c.
12.) Miyaura, N., T. Yanagi, and A. Suzuki. The Palladium-Catalyzed Cross-Coupling Reaction of Phenylboronic Acid with Haloarenes in the Presence of Bases. Synthetic Communications 11, no. (1981): 513–19. https://doi.org/10.1080/00397918108063618.
13.) Miyaura, Norio. Organoboron Compounds. Topics in Current Chemistry 219, no. (2002): 11–59.
14.) Nguyen, Hanh Nho, Xiaohua Huang, and Stephen L. Buchwald. The First General Palladium Catalyst for the Suzuki-Miyaura and Carbonyl Enolate Coupling of Aryl Arenesulfonates. Journal of the American Chemical Society 125, no. (2003): 11818–19. https://doi.org/10.1021/ja036947t.
15.) Qiao, Jennifer X., and Patrick Y. S. Lam. Copper-Promoted Carbon-Heteroatom Bond Cross-Coupling with Boronic Acids and Derivatives. Synthesis, no. (2011): 829–56. https://doi.org/10.1055/s-0030-1258379.
16.) Seiple, Ian B., Shun Su, Rodrigo A. Rodriguez, Ryan Gianatassio, Yuta Fujiwara, Adam L. Sobel, and Phil S. Baran. Direct C-H Arylation of Electron-Deficient Heterocycles with Arylboronic Acids. Journal of the American Chemical Society 132, no. (2010): 13194–96. https://doi.org/10.1021/ja1066459.
17.) Shirota, Yasuhiko, Motoi Kinoshita, Tetsuya Noda, Kenji Okumoto, and Takahiro. Ohara. A Novel Class of Emitting Amorphous Molecular Materials as Bipolar Radical Formants: 2-{4-[Bis(4-Methylphenyl)Amino]Phenyl}-5-(Dimesitylboryl)Thiophene and 2-{4-[Bis(9,9-Dimethylfluorenyl)Amino]Phenyl}-5-(Dimesitylboryl)Thiophene. Journal of the American Chemical Society 122, no. (2000): 11021–22. https://doi.org/10.1021/ja0023332.
18.) Stambuli, James P., Ryoichi Kuwano, and John F. Hartwig. Unparalleled Rates for the Activation of Aryl Chlorides and Bromides: Coupling with Amines and Boronic Acids in Minutes at Room Temperature. Angewandte Chemie, International Edition 41, no. (2002): 4746–48. https://doi.org/10.1002/anie.200290036.
19.) Tsuboyama, Akira, Hironobu Iwawaki, Manabu Furugori, Taihei Mukaide, Jun Kamatani, Satoshi Igawa, Takashi Moriyama, et al. Homoleptic Cyclometalated Iridium Complexes with Highly Efficient Red Phosphorescence and Application to Organic Light-Emitting Diode. Journal of the American Chemical Society 125, no. (2003): 12971–79. https://doi.org/10.1021/ja034732d.
20.) Wang, Dong-Hui, Masayuki Wasa, Ramesh Giri, and Jin-Quan. Yu. Pd(II)-Catalyzed Cross-Coupling of Sp3 C-H Bonds with Sp2 and Sp3 Boronic Acids Using Air as the Oxidant. Journal of the American Chemical Society 130, no. (2008): 7190–91. https://doi.org/10.1021/ja801355s.
21.) Wright, Stephen W., David L. Hageman, and Lester D. McClure. Fluoride-Mediated Boronic Acid Coupling Reactions. Journal of Organic Chemistry 59, no. (1994): 6095–97. https://doi.org/10.1021/jo00099a049.
22.) Yang, Shang-Dong, Chang-Liang Sun, Zhao Fang, Bi-Jie Li, Yi-Zhou Li, and Zhang-Jie. Shi. Palladium-Catalyzed Direct Arylation of (Hetero)Arenes with Aryl Boronic Acids. Angewandte Chemie, International Edition 47, no. (2008): 1473–76. https://doi.org/10.1002/anie.200704619.
23.) Yin, Jingjun, and Stephen L. Buchwald. A Catalytic Asymmetric Suzuki Coupling for the Synthesis of Axially Chiral Biaryl Compounds. Journal of the American Chemical Society 122, no. (2000): 12051–52. https://doi.org/10.1021/ja005622z.
24.) Zhang, Chunming, Jinkun Huang, Mark L. Trudell, and Steven P. Nolan. Palladium-Imidazol-2-Ylidene Complexes as Catalysts for Facile and Efficient Suzuki Cross-Coupling Reactions of Aryl Chlorides with Arylboronic Acids. Journal of Organic Chemistry 64, no. (1999): 3804–5. https://doi.org/10.1021/JO990554O.