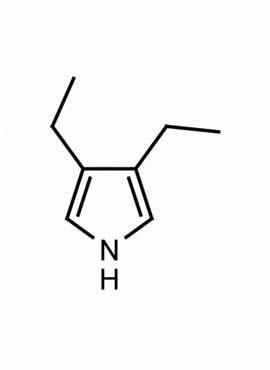
3,4-Diethylpyrrole CAS: 16200-52-5 MDL: MFCD08062571
Molecular weight: 123.2 g/mol
Molecular Formula: C8H13N
CAS Number: 16200-52-5
Storage: Store at -20 oC Under Argon, Air Sensitive!
Synonyms: 3,4-DIETHYLPYRROLE, 16200-52-5, 3,4-Diethyl-1H-pyrrole, 3,4-Diethyl-pyrrol, MFCD08062571, 3,4-diethyl pyrrole, 1H-Pyrrole,3,4-diethyl-, SCHEMBL891271
Uses: Synthesis building block, Organic Synthesis, pyrrole nitrogen heterocycle, synthesis, synthesis of porphyrins
3,4-Diethylpyrrole, is a synthetic fine chemical useful in the synthesis of pharmaceuticals, fine organic chemicals, and pyrrole beta-substituted porphyrins.
Selected References:
- Cheng, Y.; Yuan, X.; Ma, J.; Yu, S., Direct Aromatic C-H Trifluoromethylation via an Electron-Donor-Acceptor Complex. Chem. – Eur. J. 2015, 21 (23), 8355-8359.
- Dohi, T.; Ito, M.; Yamaoka, N.; Morimoto, K.; Fujioka, H.; Kita, Y., Hypervalent iodine(III): selective and efficient single-electron-transfer (SET) oxidizing agent. Tetrahedron 2009, 65 (52), 10797-10815.
- Dohi, T.; Morimoto, K.; Maruyama, A.; Kita, Y., Direct Synthesis of Bipyrroles Using Phenyliodine Bis(trifluoroacetate) with Bromotrimethylsilane. Org. Lett. 2006, 8 (10), 2007-2010.
- Ito, S.; Murashima, T.; Ono, N.; Uno, H., A new synthesis of benzoporphyrins using 4,7-dihydro-4,7-ethano-2H-isoindole as a synthon of isoindole. Chem. Commun. (Cambridge) 1998, (16), 1661-1662.
- Jaquinod, L.; Siri, O.; Khoury, R. G., Linear fused oligoporphyrins: potential molecular wires with enhanced electronic communication between bridged metal ions. Chem. Commun. (Cambridge) 1998, (12), 1261-1262.
- Kral, V.; Furuta, H.; Shreder, K.; Lynch, V.; Sessler, J. L., Protonated Sapphyrins. Highly Effective Phosphate Receptors. J. Am. Chem. Soc. 1996, 118 (7), 1595-607.
- Krayer, M.; Ptaszek, M.; Kim, H.-J.; Meneely, K. R.; Fan, D.; Secor, K.; Lindsey, J. S., Expanded Scope of Synthetic Bacteriochlorins via Improved Acid Catalysis Conditions and Diverse Dihydrodipyrrin-Acetals. J. Org. Chem. 2010, 75 (4), 1016-1039.
- Krivokapic, A.; Cowley, A. R.; Anderson, H. L., Contracted and Expanded meso-Alkynyl Porphyrinoids: from Triphyrin to Hexaphyrin. J. Org. Chem. 2003, 68 (3), 1089-1096.
- Lash, T. D., Porphyrins with exocyclic rings. Part 9. Synthesis of porphyrins by the “3 + 1” approach. J. Porphyrins Phthalocyanines 1997, 1 (1), 29-44.
- Lash, T. D.; Colby, D. A.; Szczepura, L. F., New Riches in Carbaporphyrin Chemistry: Silver and Gold Organometallic Complexes of Benzocarbaporphyrins. Inorg. Chem. 2004, 43 (17), 5258-5267.
- Liu, T.-F.; Feng, D.; Chen, Y.-P.; Zou, L.; Bosch, M.; Yuan, S.; Wei, Z.; Fordham, S.; Wang, K.; Zhou, H.-C., Topology-Guided Design and Syntheses of Highly Stable Mesoporous Porphyrinic Zirconium Metal-Organic Frameworks with High Surface Area. J. Am. Chem. Soc. 2015, 137 (1), 413-419.
- Mizutani, T.; Ema, T.; Tomita, T.; Kuroda, Y.; Ogoshi, H., Design and Synthesis of a Trifunctional Chiral Porphyrin with C2 Symmetry as a Chiral Recognition Host for Amino Acid Esters. J. Am. Chem. Soc. 1994, 116 (10), 4240-50.
- Nguyen, L. T.; Senge, M. O.; Smith, K. M., Simple Methodology for Syntheses of Porphyrins Possessing Multiple Peripheral Substituents with an Element of Symmetry. J. Org. Chem. 1996, 61 (3), 998-1003.
- Paolesse, R.; Marini, A.; Nardis, S.; Froiio, A.; Mandoj, F.; Nurco, D. J.; Prodi, L.; Montalti, M.; Smith, K. M., Novel routes to substituted 5,10,15-triarylcorroles. J. Porphyrins Phthalocyanines 2003, 7 (1), 25-36.
- Senge, M. O.; Kalisch, W. W., Synthesis and Structural Characterization of Nonplanar Tetraphenylporphyrins and Their Metal Complexes with Graded Degrees of β-Ethyl Substitution. Inorg. Chem. 1997, 36 (26), 6103-6116.
- Sessler, J. L.; Cyr, M. J.; Lynch, V.; McGhee, E.; Ibers, J. A., Synthetic and structural studies of sapphyrin, a 22-π-electron pentapyrrolic “expanded porphyrin”. J. Am. Chem. Soc. 1990, 112 (7), 2810-13.
- Sessler, J. L.; Johnson, M. R.; Lynch, V., Synthesis and crystal structure of a novel tripyrrane-containing porphyrinogen-like macrocycle. J. Org. Chem. 1987, 52 (19), 4394-7.
- Sessler, J. L.; Mody, T. D.; Hemmi, G. W.; Lynch, V., Synthesis and structural characterization of lanthanide(III) texaphyrins. Inorg. Chem. 1993, 32 (14), 3175-87.
- Sessler, J. L.; Mody, T. D.; Lynch, V., Synthesis and x-ray characterization of a uranyl(VI) Schiff base complex derived from a 2:2 condensation product of 3,4-diethylpyrrole-2,5-dicarbaldehyde and 1,2-diamino-4,5-dimethoxybenzene. Inorg. Chem. 1992, 31 (4), 529-31.
- Tang, J.; Verkade, J. G., Nonionic Superbase-Promoted Synthesis of Oxazoles and Pyrroles: Facile Synthesis of Porphyrins and α-C-Acyl Amino Acid Esters. J. Org. Chem. 1994, 59 (25), 7793-802.