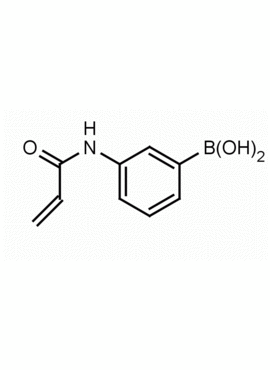
3-Acrylamidophenylboronic acid CAS: 99349-68-5 MDL: MFCD09025755
Molecular weight: 115.97 g/mol
Molecular Formula: C9H10BNO3
CAS Number: 99349-68-5
Storage: Store at –20 °C , under dry conditions.
Synonyms: 99349-68-5, 3-Acrylamidophenylboronic acid, (3-Acrylamidophenyl)boronic acid, (M-Acrylamidophenyl)boronic acid, [3-(prop-2-enoylamino)phenyl]boronic acid
[3-(prop-2-enamido)phenyl]boronic acid, 3-(Acrylamido)phenylboronic acid
ACMC-209scf, SCHEMBL235432
Uses: Transition Metal Coupling Reactions
Selected References
Boronic Acid Derivative Coupling Reactions
1.) Altenhoff, Gereon, Richard Goddard, Christian W. Lehmann, and Frank. Glorius. Sterically Demanding, Bioxazoline-Derived N-Heterocyclic Carbene Ligands with Restricted Flexibility for Catalysis. Journal of the American Chemical Society 126, no. (2004): 15195–201. https://doi.org/10.1021/ja045349r.
2.) Arvela, Riina K., Nicholas E. Leadbeater, Michael S. Sangi, Victoria A. Williams, Patricia Granados, and Robert D. Singer. A Reassessment of the Transition-Metal Free Suzuki-Type Coupling Methodology. Journal of Organic Chemistry 70, no. (2005): 161–68. https://doi.org/10.1021/jo048531j.
3.) Billingsley, Kelvin, and Stephen L. Buchwald. Highly Efficient Monophosphine-Based Catalyst for the Palladium-Catalyzed Suzuki-Miyaura Reaction of Heteroaryl Halides and Heteroaryl Boronic Acids and Esters. Journal of the American Chemical Society 129, no. (2007): 3358–66. https://doi.org/10.1021/ja068577p.
4.) Billingsley, Kelvin L., Kevin W. Anderson, and Stephen L. Buchwald. A Highly Active Catalyst for Suzuki-Miyaura Cross-Coupling Reactions of Heteroaryl Compounds. Angewandte Chemie, International Edition 45, no. (2006): 3484–88. https://doi.org/10.1002/anie.200600493.
5.) Gillis, Eric P., and Martin D. Burke. A Simple and Modular Strategy for Small Molecule Synthesis: Iterative Suzuki-Miyaura Coupling of B-Protected Haloboronic Acid Building Blocks. Journal of the American Chemical Society 129, no. (2007): 6716–17. https://doi.org/10.1021/ja0716204.
6.) Herradura, Prudencio S., Kathleen A. Pendola, and R. Kiplin. Guy. Copper-Mediated Cross-Coupling of Aryl Boronic Acids and Alkyl Thiols. Organic Letters 2, no. (2000): 2019–22. https://doi.org/10.1021/ol005832g.
7.) Kataoka, Noriyasu, Quinetta Shelby, James P. Stambuli, and John F. Hartwig. Air Stable, Sterically Hindered Ferrocenyl Dialkylphosphines for Palladium-Catalyzed C-C, C-N, and C-O Bond-Forming Cross-Couplings. Journal of Organic Chemistry 67, no. (2002): 5553–66. https://doi.org/10.1021/jo025732j.
8.) Kinzel, Tom, Yong Zhang, and Stephen L. Buchwald. A New Palladium Precatalyst Allows for the Fast Suzuki-Miyaura Coupling Reactions of Unstable Polyfluorophenyl and 2-Heteroaryl Boronic Acids. Journal of the American Chemical Society 132, no. (2010): 14073–75. https://doi.org/10.1021/ja1073799.
9.) Knapp, David M., Eric P. Gillis, and Martin D. Burke. A General Solution for Unstable Boronic Acids: Slow-Release Cross-Coupling from Air-Stable MIDA Boronates. Journal of the American Chemical Society 131, no. (2009): 6961–63. https://doi.org/10.1021/ja901416p.
10.) Liebeskind, Lanny S., and Jiri. Srogl. Thiol Ester-Boronic Acid Coupling. A Mechanistically Unprecedented and General Ketone Synthesis. Journal of the American Chemical Society 122, no. (2000): 11260–61. https://doi.org/10.1021/ja005613q.
11.) McGuinness, David S., and Kingsley J. Cavell. Donor-Functionalized Heterocyclic Carbene Complexes of Palladium(II): Efficient Catalysts for C-C Coupling Reactions. Organometallics 19, no. (2000): 741–48. https://doi.org/10.1021/om990776c.
12.) Miyaura, N., T. Yanagi, and A. Suzuki. The Palladium-Catalyzed Cross-Coupling Reaction of Phenylboronic Acid with Haloarenes in the Presence of Bases. Synthetic Communications 11, no. (1981): 513–19. https://doi.org/10.1080/00397918108063618.
13.) Miyaura, Norio. Organoboron Compounds. Topics in Current Chemistry 219, no. (2002): 11–59.
14.) Nguyen, Hanh Nho, Xiaohua Huang, and Stephen L. Buchwald. The First General Palladium Catalyst for the Suzuki-Miyaura and Carbonyl Enolate Coupling of Aryl Arenesulfonates. Journal of the American Chemical Society 125, no. (2003): 11818–19. https://doi.org/10.1021/ja036947t.
15.) Qiao, Jennifer X., and Patrick Y. S. Lam. Copper-Promoted Carbon-Heteroatom Bond Cross-Coupling with Boronic Acids and Derivatives. Synthesis, no. (2011): 829–56. https://doi.org/10.1055/s-0030-1258379.
16.) Seiple, Ian B., Shun Su, Rodrigo A. Rodriguez, Ryan Gianatassio, Yuta Fujiwara, Adam L. Sobel, and Phil S. Baran. Direct C-H Arylation of Electron-Deficient Heterocycles with Arylboronic Acids. Journal of the American Chemical Society 132, no. (2010): 13194–96. https://doi.org/10.1021/ja1066459.
17.) Shirota, Yasuhiko, Motoi Kinoshita, Tetsuya Noda, Kenji Okumoto, and Takahiro. Ohara. A Novel Class of Emitting Amorphous Molecular Materials as Bipolar Radical Formants: 2-{4-[Bis(4-Methylphenyl)Amino]Phenyl}-5-(Dimesitylboryl)Thiophene and 2-{4-[Bis(9,9-Dimethylfluorenyl)Amino]Phenyl}-5-(Dimesitylboryl)Thiophene. Journal of the American Chemical Society 122, no. (2000): 11021–22. https://doi.org/10.1021/ja0023332.
18.) Stambuli, James P., Ryoichi Kuwano, and John F. Hartwig. Unparalleled Rates for the Activation of Aryl Chlorides and Bromides: Coupling with Amines and Boronic Acids in Minutes at Room Temperature. Angewandte Chemie, International Edition 41, no. (2002): 4746–48. https://doi.org/10.1002/anie.200290036.
19.) Tsuboyama, Akira, Hironobu Iwawaki, Manabu Furugori, Taihei Mukaide, Jun Kamatani, Satoshi Igawa, Takashi Moriyama, et al. Homoleptic Cyclometalated Iridium Complexes with Highly Efficient Red Phosphorescence and Application to Organic Light-Emitting Diode. Journal of the American Chemical Society 125, no. (2003): 12971–79. https://doi.org/10.1021/ja034732d.
20.) Wang, Dong-Hui, Masayuki Wasa, Ramesh Giri, and Jin-Quan. Yu. Pd(II)-Catalyzed Cross-Coupling of Sp3 C-H Bonds with Sp2 and Sp3 Boronic Acids Using Air as the Oxidant. Journal of the American Chemical Society 130, no. (2008): 7190–91. https://doi.org/10.1021/ja801355s.
21.) Wright, Stephen W., David L. Hageman, and Lester D. McClure. Fluoride-Mediated Boronic Acid Coupling Reactions. Journal of Organic Chemistry 59, no. (1994): 6095–97. https://doi.org/10.1021/jo00099a049.