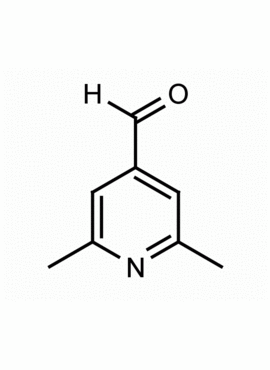
2,6-Dimethylpyridine-4-carbaldehyde
Talk with our team about available sizes and pricing!
MDL: MFCD06410687
Molecular Formula: C8H9NO
CAS Number: 18206-06-9
Storage: Store at 2-8 Co, under dry conditions.
Synonyms: 2,6-Dimethylisonicotinaldehyde, 18206-06-9, 2,6-dimethylpyridine-4-carbaldehyde, 2,6-Dimethylpyridine-4-carboxaldehyde, 4-formyl-2,6-dimethylpyridine, 2,6-dimethyl-4-pyridinecarboxaldehyde, 4-Pyridinecarboxaldehyde, 2,6-dimethyl-, 4 Pyridinecarboxaldehyde,2,6-dimethyl-
Uses: Synthesis building block, Organic Synthesis, pyridine nitrogen heterocycle, synthesis, aldehyde reactions
2,6-Dimethylpyridine-4-carbaldehyde, is a synthetic fine chemical useful in the synthesis of pharmaceuticals and fine organic chemicals.
Selected References:
1-Substituted 4-Aryl-5-pyridinylimidazoles: A New Class of Cytokine Suppressive Drugs with Low 5-Lipoxygenase and Cyclooxygenase Inhibitory Potency, Boehm, Jeffrey C., Smietana, Juanita M., Sorenson, Margaret E., Garigipati, Ravi S., Gallagher, Timothy F., Sheldrake, Peter L., Bradbeer, Jeremy, Badger, Alison M., Laydon, Jeffrey T., et al., Journal of Medicinal Chemistry (1996), 39(20), 3929-3937. DOI:10.1021/JM960415O
Optical pH Sensor Covering the Range from pH 0-14 Compatible with Mobile-Device Readout and Based on a Set of Rationally Designed Indicator Dyes, Gotor, Raul, Ashokkumar, Pichandi, Hecht, Mandy, Keil, Karin, Rurack, Knut, Analytical Chemistry (Washington, DC, United States) (2017), 89(16), 8437-8444., DOI:10.1021/acs.analchem.7b01903
Synthesis and pH-sensitive luminescence of bis-terpyridyl iridium(III) complexes incorporating pendent pyridyl groups, Arm, Kathryn J., Leslie, Wendy, Williams, J. A. Gareth, Inorganica Chimica Acta (2006), 359(4), 1222-1232. DOI:10.1016/j.ica.2005.09.021
Selective photosensitization through an AND logic response: optimization of the pH and glutathione response of activatable photosensitizers, Erbas-Cakmak, Sundus, Cakmak, Fatma Pir, Topel, Seda Demirel, Uyar, Taha Bilal, Akkaya, Engin U., Chemical Communications (Cambridge, United Kingdom) (2015), 51(61), 12258-12261. DOI:10.1039/C5CC01261A
https://www.ncbi.nlm.nih.gov/pubmed/26134889
Preparation of azolylmethylaminotetrahydroquinolines and related compounds as chemokine receptor binding agents., Bridger, Gary, Skerlj, Renato, Kaller, Al, Harwig, Curtis, Bogucki, David, Wilson, Trevor R., Crawford, Jason, McEachern, Ernest J., Atsma, Bem, Nan, Siqiao, et al, PCT Int. Appl. (2002), WO 2002022600 A2 20020321
Preparation of substituted pyrazolo[1,5-a]pyridine compounds as RET kinase inhibitors, Andrews, Steven W., Aronow, Sean, Blake, James F., Brandhuber, Barbara J., Cook, Adam, Haas, Julia, Jiang, Yutong, Kolakowski, Gabrielle R., McFaddin, Elizabeth A., McKenney, Megan L., et al, PCT Int. Appl. (2018), WO 2018071447 A1 20180419
Preparation and antibacterial activity of tricyclic carbapenem compounds, Rossi, Tino, Andreotti, Daniele, Tedesco, Giovanna, Tarsi, Luca, Ratti, Emiliangelo, Feriani, Aldo, Pizzi, Domenica Antonia, Gaviraghi, Giovari, Biondi, Stefano, Finizia, Gabriella, PCT Int. Appl. (1998), WO 9821210 A1 19980522,
Preparation of substituted 2-phenyl-3H-quinazolin-4-ones and analogs as antiinflammatory and cardiovascular agents, Hansen, C. Henrik, PCT Int. Appl. (2010), WO 2010106436 A2 20100923
Derivatives of 2,4,6-collidine and 2,4-lutidine, Mathes, Wilhelm, Sauermilch, Walter, Chemische Berichte (1955), 88, 1276-83.
Preparation of substituted diazaspirononanes as OGA inhibitors, Bartolome-Nebreda, Jose Manuel, Trabanco-Suarez, Andres Avelino, Martinez Viturro, Carlos Manuel, PCT Int. Appl. (2018), WO 2018141984 A1 20180809