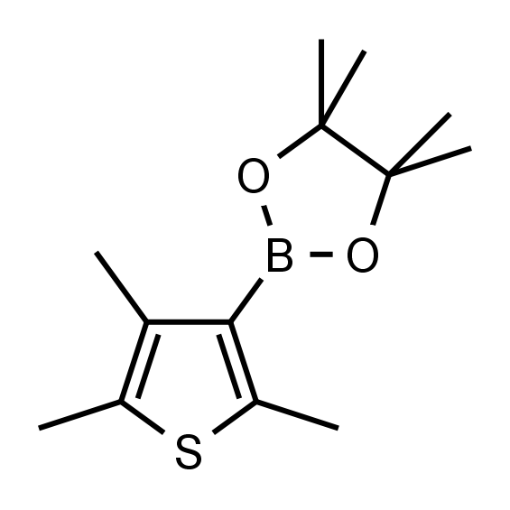
Name: (2,4,5-Trimethylthiophen-3-yl)boronic acid pinacol ester
Molecular Formula: C13H21BO2S
CAS#: 2064117-97-9
SMILES: CC1=C(C)C(B2OC(C)(C)C(C)(C)O2)=C(C)S1
MDL#: MFCD26404006
Catalog#: T36115
Molecular weight: 252.18 g/mol
Other names:
- 4,4,5,5-Tetramethyl-2-(2,4,5-trimethylthiophen-3-yl)-1,3,2-dioxaborolane
Fields of Interest: Organic Synthesis, Suzuki-Miyaura coupling reactions
Background & Applications: Sulfur-containing heterocycles like (2,4,5-Trimethylthiophen-3-yl)boronic acid pinacol ester are a crucial class of compounds in organic chemistry due to their diverse structures and wide-ranging applications in various fields such as medicinal chemistry, agrochemicals, and materials science. These heterocycles incorporate sulfur atoms into their ring structures, which can significantly influence the chemical properties and biological activities of the compounds.
The most common catalysts used in Suzuki coupling reactions are palladium based; however, palladium is expensive, rare, and its presence in APIs is limited to a very low ppm by stringent regulatory requirements. New discoveries are making it possible to replace palladium with other less toxic and more available transition metals such as cobalt when the boronic acid is used as its neopentyl ester.
Appearance: Clear liquid
Purity: 98%
Storage: Store @ 3-5 °C under inert atmosphere
Solubility:
- Soluble in organic solvents such as ethyl ether or dioxane
Literature:
- Kotha, S.; Khedkar, P. Design of Synthetic Strategies towards Sulfur Containing Scaffolds. Synthesis 2024. https://doi.org/10.1055/s-0043-1775393.