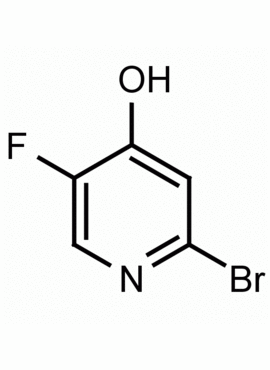
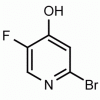
2-Bromo-5-fluoro-4-hydroxypyridine 2-Bromo-5-fluoropyridin-4-ol CAS: 1196152-88-1 MDL: MFCD13189884
Molecular weight: 199.19 g/mol
Molecular Formula: C5H3BrFNO
CAS Number: 1196152-88-1
Storage: Store at 2-8 Co, under dry conditions.
Synonyms: 2-Bromo-5-fluoropyridin-4-ol, 1196152-88-1, 2-Bromo-5-fluoro-4-hydroxypyridine, 2-bromo-5-fluoro-1H-pyridin-4-one, SCHEMBL16033299
Uses: Synthesis building block, Organic Synthesis, pyridine nitrogen heterocycle, synthesis, bromine reactions
2-Bromo-5-fluoro-4-hydroxypyridine, is a synthetic fine chemical useful in the synthesis of pharmaceuticals and fine organic chemicals.
Selected References:
Aryl Bromide Coupling Reactions Selected References:
Ackerman, Laura K. G., Matthew M. Lovell, and Daniel J. Weix. Multimetallic Catalysed Cross-Coupling of Aryl Bromides with Aryl Triflates. Nature (London, United Kingdom) 524, (2015): 454–57. https://doi.org/10.1038/nature14676.
Atwater, Bruce, Nalin Chandrasoma, David Mitchell, Michael J. Rodriguez, Matthew Pompeo, Robert D. J. Froese, and Michael G. Organ. The Selective Cross-Coupling of Secondary Alkyl Zinc Reagents to Five-Membered-Ring Heterocycles Using Pd-PEPPSI-IHeptCl. Angewandte Chemie, International Edition 54, (2015): 9502–6. https://doi.org/10.1002/anie.201503941.
Burda, Edyta, Werner Hummel, and Harald. Groeger. Modular Chemoenzymatic One-Pot Syntheses in Aqueous Media: Combination of a Palladium-Catalyzed Cross-Coupling with an Asymmetric Biotransformation. Angewandte Chemie, International Edition 47, (2008): 9551–54. https://doi.org/10.1002/anie.200801341.
Clayden, Jonathan, and Ulrich. Hennecke. α-Pyridylation of Chiral Amines via Urea Coupling, Lithiation and Rearrangement. Organic Letters 10, (2008): 3567–70. https://doi.org/10.1021/ol801332n.
Esumi, Tomoyuki, Gouki Makado, Haifeng Zhai, Yasuhiro Shimizu, Yasuhide Mitsumoto, and Yoshiyasu. Fukuyama. Efficient Synthesis and Structure-Activity Relationship of Honokiol, a Neurotrophic Biphenyl-Type Neolignan. Bioorganic & Medicinal Chemistry Letters 14, (2004): 2621–25. https://doi.org/10.1016/j.bmcl.2004.02.067.
Fernandez-Rodriguez, Manuel A., and John F. Hartwig. A General, Efficient, and Functional-Group-Tolerant Catalyst System for the Palladium-Catalyzed Thioetherification of Aryl Bromides and Iodides. Journal of Organic Chemistry 74, (2009): 1663–72. https://doi.org/10.1021/jo802594d.
Goossen, Lukas J., Guojun Deng, and Laura M. Levy. Synthesis of Biaryls via Catalytic Decarboxylative Coupling. Science (Washington, DC, United States) 313, (2006): 662–64. https://doi.org/10.1126/science.1128684.
Koch, Karl Heinz, and Klaus. Muellen. Polyarylenes and Poly(Arylenevinylene)s. V. Synthesis of Tetraalkyl-Substituted Oligo(1,4-Naphthylene)s and Cyclization to Soluble Oligo(Peri-Naphthylene)s. Chemische Berichte 124, (1991): 2091–2100. https://doi.org/10.1002/cber.19911240935.
Liang, Lan-Chang, Pin-Shu Chien, and Mei-Hui. Huang. Catalytic Suzuki Coupling Reactions by Amido Phosphine Complexes of Palladium. Organometallics 24, (2005): 353–57. https://doi.org/10.1021/om0492395.
Liu, Qing-Xiang, Wei Zhang, Xiao-Jun Zhao, Zhi-Xiang Zhao, Meng-Chao Shi, and Xiu-Guang. Wang. NHC PdII Complex Bearing 1,6-Hexylene Linker: Synthesis and Catalytic Activity in the Suzuki-Miyaura and Heck-Mizoroki Reactions. European Journal of Organic Chemistry 2013, (2013): 1253–61. https://doi.org/10.1002/ejoc.201200954.
Marziale, Alexander N., Stefan H. Faul, Thomas Reiner, Sven Schneider, and Jorg. Eppinger. Facile Palladium-Catalyzed Suzuki-Miyaura Coupling in Air and Water at Ambient Temperature. Green Chemistry 12, (2010): 35–38. https://doi.org/10.1039/B915436A.
Matsuda, Takanori, Masanori Shigeno, and Masahiro. Murakami. Palladium-Catalyzed Sequential Carbon-Carbon Bond Cleavage/Formation Producing Arylated Benzolactones. Organic Letters 10, (2008): 5219–21. https://doi.org/10.1021/ol802218a.
Molander, Gary A., and Maria. Ribagorda. Expanding Organoboron Chemistry: Epoxidation of Potassium Organotrifluoroborates. Journal of the American Chemical Society 125, (2003): 11148–49. https://doi.org/10.1021/ja0351140.
Pabst, Gunther R., Oliver C. Pfuller, and Jurgen. Sauer. The New and Simple ‘LEGO’ System: Synthesis and Reactions of Ruthenium(II) Complexes. Tetrahedron 55, (1999): 8045–64. https://doi.org/10.1016/S0040-4020(99)00422-6.
Patil, Siddappa A., Chia-Ming Weng, Po-Cheng Huang, and Fung-E. Hong. Convenient and Efficient Suzuki-Miyaura Cross-Coupling Reactions Catalyzed by Palladium Complexes Containing N,N,O-Tridentate Ligands. Tetrahedron 65, (2009): 2889–97. https://doi.org/10.1016/j.tet.2009.02.017.
Uno, Brice E., Eric P. Gillis, and Martin D. Burke. Vinyl MIDA Boronate: A Readily Accessible and Highly Versatile Building Block for Small Molecule Synthesis. Tetrahedron 65, (2009): 3130–38. https://doi.org/10.1016/j.tet.2008.11.010.
Waybright, Shane M., Kanika McAlpine, Matthew Laskoski, Mark D. Smith, and Uwe H. F. Bunz. Organometallic Dendrimers Based on (Tetraphenylcyclobutadiene)Cyclopentadienylcobalt Modules. Journal of the American Chemical Society 124, (2002): 8661–66. https://doi.org/10.1021/ja026462p.
Xiong, Zhengchang, Nengdong Wang, Mingji Dai, Ang Li, Jiahua Chen, and Zhen. Yang. Synthesis of Novel Palladacycles and Their Application in Heck and Suzuki Reactions under Aerobic Conditions. Organic Letters 6, (2004): 3337–40. https://doi.org/10.1021/ol048749s.
Zhang, Qingling, Thomas P. Russell, and Todd. Emrick. Synthesis and Characterization of CdSe Nanorods Functionalized with Regioregular Poly(3-Hexylthiophene). Chemistry of Materials 19, (2007): 3712–16. https://doi.org/10.1021/cm070603a.
Zhou, Wen-Jun, Ke-Hu Wang, and Jin-Xian. Wang. Pd(PPh3)4-PEG 400 Catalyzed Protocol for the Atom-Efficient Stille Cross-Coupling Reaction of Organotin with Aryl Bromides. Journal of Organic Chemistry 74, (2009): 5599–5602. https://doi.org/10.1021/jo9005206.