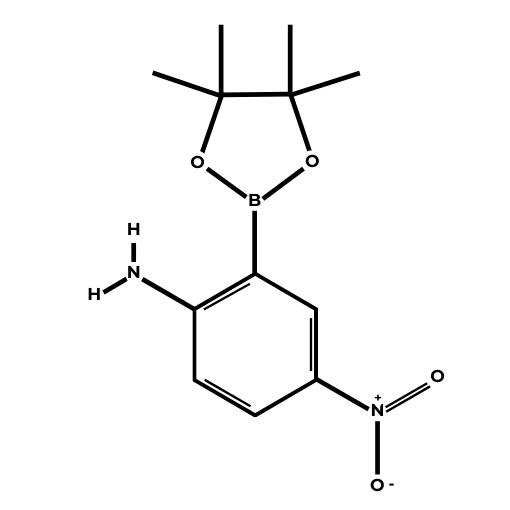
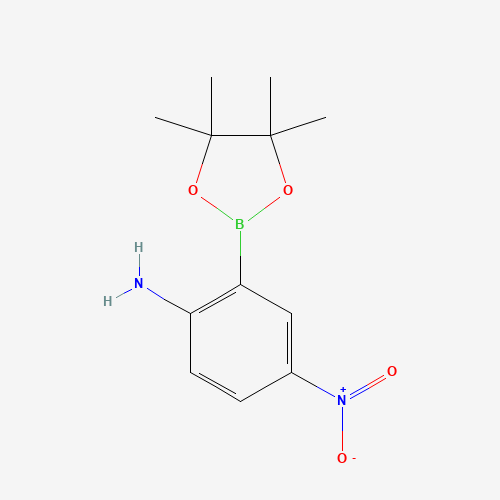
Name: 2-Amino-5-nitrophenylboronic acid pinacol ester
Molecular Formula: C12H17BN2O4
CAS#: 1351337-48-8
SMILES: B1(OC(C(O1)(C)C)(C)C)C2=C(C=CC(=C2)[N+](=O)[O-])N
MDL#: MFCD22493583
Catalog#: A36099
Molecular weight: 264.09 g/mol
Other names:
- 4-Nitro-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)aniline
Fields of Interest: Suzuki coupling / Suzuki reaction / Suzuki-Miyaura coupling, organic synthesis
Background & Applications:
2-Amino-5-nitrophenylboronic acid pinacol ester (CAS 1351337-48-8) is a compound used in cross-coupling reactions such as Suzuki coupling (also called Suzuki cross-coupling, Suzuki-Miyaura coupling, or suzuki reaction). This type of reaction is a powerful tool in organic chemistry for forming carbon-carbon bonds and is widely used for synthesizing various chemical compounds.
In the context of cross-coupling, boronic acids and their esters, like this pinacol ester, are often used as reagents. They are known for their stability and reactivity which make them suitable for transmetalation steps in the coupling process (1). The Suzuki-Miyaura coupling, in particular, is favored for its mild conditions and functional group tolerance, making it applicable to a wide range of substrates (2).
2-Amino-5-nitrophenylboronic acid pinacol ester would typically undergo a reaction with an appropriate palladium catalyst and a halide or triflate partner under basic conditions.
If you’re looking to utilize this compound in a laboratory setting, it’s important to consider the specific conditions and catalysts that will best suit your synthetic goals. The literature provided below can offer various protocols and examples of successful cross-coupling reactions using similar boronic acid esters, which can serve as a guide and inspiration for your experiments.
Appearance: light orange solid
Purity: 95%
Storage: Store @ 3-5 °C and under Argon/Nitrogen
Literature:
- Catalytic protodeboronation of pinacol boronic esters: formal anti-Markovnikov hydromethylation of alkenes – Chemical Science (RSC Publishing) DOI:10.1039/C9SC02067E
- Selection of boron reagents for Suzuki–Miyaura coupling – Chemical Society Reviews (RSC Publishing) DOI:10.1039/C3CS60197H
- Galenko, Ekaterina E. and Zanakhov, Timur O. and Novikov, Mikhail S. and Khlebnikov, Alexander. Pd-Catalyzed Heteroannulation of Isoxazoles: Convergent Synthesis of Isoxazolo[5,4-C]Quinolines. Available at SSRN: https://ssrn.com/abstract=4227821 or http://dx.doi.org/10.2139/ssrn.4227821
- Boronic Acids and Their Derivatives in Medicinal Chemistry: Synthesis and Biological Applications (mdpi.com). Silva, M.P.; Saraiva, L.; Pinto, M.; Sousa, M.E. Boronic Acids and Their Derivatives in Medicinal Chemistry: Synthesis and Biological Applications. Molecules 2020, 25, 4323. https://doi.org/10.3390/molecules25184323
-
Milton R. Smith III, Ranjana Bisht, Chabush Haldar, Gajanan Pandey, Jonathan E. Dannatt, Behnaz Ghaffari, Robert E. Maleczka Jr.*, and Buddhadeb Chattopadhyay*. Achieving High Ortho Selectivity in Aniline C–H Borylations by Modifying Boron Substituents. ACS Catal. 2018, 8, 7, 6216–6223. Available at ACS Publications: https://pubs.acs.org/doi/10.1021/acscatal.8b00641 or from Research Gate https://www.researchgate.net/publication/325056283_Achieving_High_Ortho_Selectivity_in_Aniline_C-H_Borylations_by_Modifying_Boron_Substituents
Full color molecular structure image provided by PubChem:
- PubChem Identifier: CID 76848364
- URL: https://pubchem.ncbi.nlm.nih.gov/compound/76848364