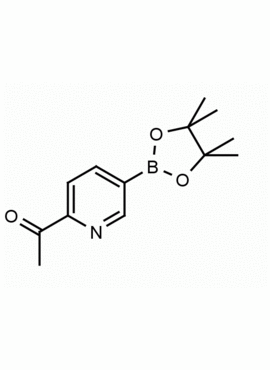
2-Acetylpyridine-5-boronic acid pinacol ester 1-(5-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-2-yl)ethanone CAS: 741709-59-1 MDL: MFCD12923434
Molecular weight: 247.098 g/mol
Molecular Formula: C13H18BNO3
CAS Number: 741709-59-1
Storage: Store at 2-8 Co, under dry conditions.
Synonyms: 2-Acetylpyridine-5-boronic acid pinacol ester, 1-(5-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-2-yl)ethanone, 741709-59-1, MFCD12923434
Uses: Synthesis building block, Organic Synthesis, Transition Metal Coupling
2-Acetylpyridine-5-boronic acid pinacol ester is a synthetic specialty chemical useful in the synthesis of pharmaceuticals and specialty organic chemicals.
Pinacol Ester Derivative Coupling Reactions
1.) Palladium(0)-Catalyzed Cross-Cross-Coupling Reaction of Alkoxydiboron with Haloarenes: A Direct Procedure for Arylboronic Esters, Ishiyama, Tatsuo, Murata, Miki, Miyaura, Norio, Journal of Organic Chemistry (1995), 60(23), 7508-10. DOI:10.1021/jo00128a024
2.) Cross Coupling Reactions of Chiral Secondary Organoboronic Esters With Retention of Configuration, Imao, Daisuke, Glasspoole, Ben W., Laberge, Veronique S., Crudden, Cathleen M., Journal of the American Chemical Society (2009), 131(14), 5024-5025. DOI:10.1021/ja8094075
3.) Palladium-Catalyzed Cross-Coupling Reaction of Bis(pinacolato)diboron with 1-Alkenyl Halides or Triflates: Convenient Synthesis of Unsymmetrical 1,3-Dienes via the Borylation-Coupling Sequence, Takagi, Jun, Takahashi, Kou, Ishiyama, Tatsuo, Miyaura, Norio, Journal of the American Chemical Society (2002), 124(27), 8001-8006. DOI:10.1021/ja0202255
4.) Functionalized olefin cross-coupling to construct carbon-carbon bonds, Lo, Julian C., Gui, Jinghan, Yabe, Yuki, Pan, Chung-Mao, Baran, Phil S., Nature (London, United Kingdom) (2014), 516(7531), 343-348. DOI:10.1038/nature14006.
5.) The Synthesis of Highly Substituted Isoxazoles by Electrophilic Cyclization: An Efficient Synthesis of Valdecoxib, Waldo, Jesse P., Larock, Richard C., Journal of Organic Chemistry (2007), 72(25), 9643-9647. DOI:10.1021/jo701942e.
6.) Arenes to anilines and aryl ethers by sequential iridium-catalyzed borylation and copper-catalyzed coupling, Tzschucke, C. Christoph, Murphy, Jaclyn M., Hartwig, John F., Organic Letters (2007), 9(5), 761-764. DOI:10.1021/ol062902w
7.) A synthesis of allyboronates via the palladium(0)-catalyzed cross-coupling reaction of bis(pinacolato)diboron with allylic acetates, Ishiyama, Tatsuo, Ahiko, Taka-aki, Miyaura, Norio, Tetrahedron Letters (1996), 37(38), 6889-6892. DOI:10.1016/0040-4039(96)01505-5
8.) Rapid synthesis of 3-amino-imidazopyridines by a microwave-assisted four-component coupling in one pot, DiMauro, Erin F., Kennedy, Joseph M., Journal of Organic Chemistry (2007), 72(3), 1013-1016, DOI:10.1021/jo0622072
9.) Iron-Catalyzed C(sp2)-H Bond Functionalization with Organoboron Compounds, Shang, Rui, Ilies, Laurean, Asako, Sobi, Nakamura, Eiichi, Journal of the American Chemical Society (2014), 136(41), 14349-14352. DOI:10.1021/ja5070763
10.) Copper-Promoted Coupling of Vinyl Boronates and Alcohols: A Mild Synthesis of Allyl Vinyl Ethers, Shade, Ryan E., Hyde, Alan M., Olsen, John-Carl, Merlic, Craig A., Journal of the American Chemical Society (2010), 132(4), 1202-1203. DOI:10.1021/ja907982w