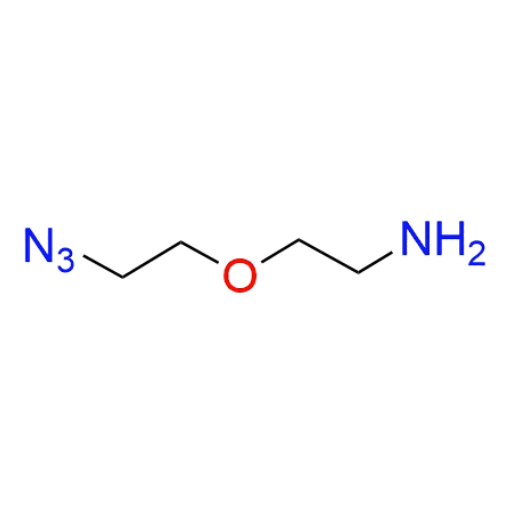
Name: 2-(2-azidoethoxy)ethan-1-amine (99% purity)
Molecular Formula: C₄H₁₀N₄O
CAS#: 464190-91-8
SMILES: NCCOCCN=[N⁺]=[N⁻]
MDL#: MFCD23726611
Catalog#: AMTGC780-EA21-99
Molecular weight: 130.15g/mol
Other names:
- Amino-PEG1-azide
- Azido-PEG1-amine
Fields of Interest: PEGylating Reagent, Medicinal chemistry, organic synthesis
Background & Applications:
Background
2-(2-azidoethoxy)ethan-1-amine (also known as Azido-PEG1-amine) (CAS 464190-91-8) is a compact, heterobifunctional azide-functionalized PEG linker featuring a PEG1 spacer with a terminal azide group and a primary amine. Despite its short chain length, the PEG unit provides improved hydrophilicity and flexibility compared to purely aliphatic linkers, while maintaining minimal spacer distance. The azide functionality enables efficient bioorthogonal click chemistry, including CuAAC and strain-promoted azide–alkyne cycloaddition, and the primary amine allows straightforward coupling to activated carboxylic acids, NHS esters, and isocyanates. This small, dual-functional linker is a valuable building block within a versatile portfolio of functionalized PEGs designed for precise and predictable molecular conjugation.
Applications
2-(2-azidoethoxy)ethan-1-amine is commonly used in bioconjugation, drug delivery, and materials science applications where minimal linker length and orthogonal reactivity are required. Typical uses include site-specific attachment of small molecules, peptides, or probes via click chemistry followed by amide bond formation, preparation of multifunctional intermediates, and surface or nanoparticle modification. As part of a comprehensive functionalized PEG product line, this azido-amine PEG supports modular design strategies for pharmaceutical research, diagnostics, and advanced materials development where tight spatial control is critical.
- The amine terminus readily reacts with carboxylic acids, activated esters (e.g., NHS esters), isocyanates, or other electrophiles to form covalent bonds.
- The azide group is a classic “click chemistry” handle, participating in copper-catalyzed azide-alkyne cycloaddition (CuAAC) or strain-promoted azide-alkyne cycloaddition (SPAAC) with alkynes and related partners.
- This makes the compound useful in bioconjugation, PEGylation, linker synthesis, PROTAC design, antibody–drug conjugates (ADCs), and surface modification applications.
Appearance: Colorless liquid
Purity: 99%
Storage: -20°C
Solubility: Soluble in water, DMSO, DMF, DCM and other polar organic solvents.
Literature:
PEG-based linkers like Azido-PEG1-amine are widely used in click chemistry and drug-conjugate synthesis research. PROTACs and ADCs frequently utilize PEG spacers of varying lengths to tune solubility and physical properties.
Journal of the American Chemical Society, 2010, vol. 132, # 13, p. 4550 – 4551, https://doi.org/10.1021/ja100746d
Journal of Porphyrins and Phthalocyanines, 2013, vol. 17, # 1-2, p. 104 117, https://doi.org/10.1142/S1088424612501350