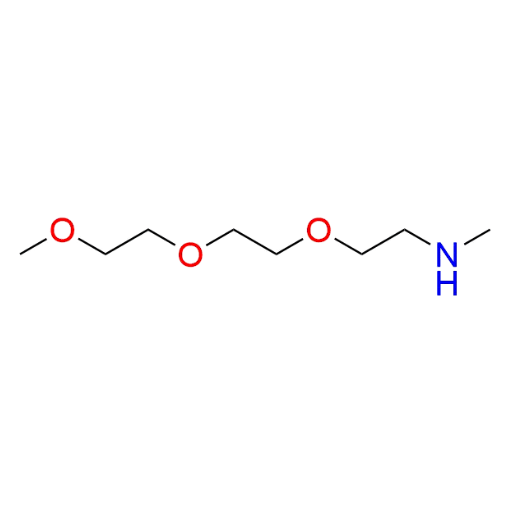
Name: 2-(2-(2-Methoxyethoxy)ethoxy)-N-methylethan-1-amine (98% purity)
Molecular Formula: C8H19NO3
CAS#: 51952-13-7
SMILES: CNCCOCCOCCOC
MDL#: MFCD19672617
Catalog#: AMTGC615-MA20
Molecular weight: 177.24 g/mol
Other names:
- 2,5,8-Trioxa-11-azadodecane
- mPEG3-NHMe
- Ethanamine, 2-[2-(2-methoxyethoxy)ethoxy]-N-methyl-1-amine
Fields of Interest: PEGylating reagent, medicinal chemistry, organic synthesis
Background & Applications:
Background
2-(2-(2-Methoxyethoxy)ethoxy)-N-methylethan-1-amine (CAS 51952-13-7) is a monofunctional amine-functionalized PEG derivative based on a PEG3 backbone, featuring a terminal secondary amine and a methoxy-capped end. The polyethylene glycol chain provides hydrophilicity, flexibility, and compatibility with aqueous and biological environments. The secondary amine functionality enables selective coupling to activated electrophiles, while the methoxy terminus remains non-reactive, allowing controlled, single-point conjugation. This compound serves as a versatile intermediate within a comprehensive portfolio of functionalized PEGs designed for predictable and modular molecular functionalization.
Applications
This methyl-substituted amine PEG is commonly used in bioconjugation, drug delivery, and materials science applications where monofunctional reactivity and defined PEG spacing are required. Typical uses include PEGylation of small molecules and polymers to improve solubility and stability, preparation of functional intermediates, and surface or nanoparticle modification. As part of a robust functionalized PEG product line, 2-(2-(2-Methoxyethoxy)ethoxy)-N-methylethan-1-amine supports modular design strategies in pharmaceutical research, diagnostics, biomaterials, and specialty chemical development.
Appearance: Colorless Liquid
Purity: 98%
Storage: 0-3 °C for long term storage
Solubility: Soluble in polar organic solvents such as methanol, ethanol, acetonitrile, DMSO, DMF, and miscible with water due to its PEG backbone.
Literature:
Widely mentioned in supplier datasheets and PEG amine reagent catalogs as a reagent for linker synthesis and surface functionalization; usage contexts include PEGylation and spacer construction in research chemistry publications.
- Journal of Organometallic Chemistry, 1974, vol. 66, p. 209 – 217, https://doi.org/10.1016/S0022-328X(00)91484-0
- Organic Letters, 2012, vol. 14, # 1, p. 330 – 333, https://doi.org/10.1021/ol203074p
![Molecular structure for PEG 2-[2-(2-Aminoethoxy)ethoxy]ethanol (CAS 6338-55-2) from Frontier Specialty Chemicals.](https://frontierspecialtychemicals.com/wp-content/uploads/2026/02/AMTGC598-AE20-Square-510x510-1.png)