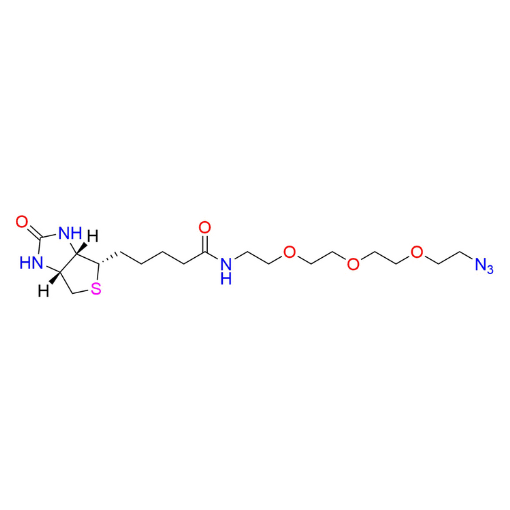
Name: 2-(2-(2-Azidoethoxy)ethoxy)-N-methylethanamine (99% purity)
Molecular Formula: C18H32N6O5S
CAS#: 875770-34-6
SMILES: O=C1N[C@@]([H])([C@]([H])(CS2)N1)[C@@H]2CCCCC(NCCOCCOCCOCCN=[N+]=[N-])=O
MDL#: MFCD20134145
Catalog#: AMTGC842-BA21-99
Molecular weight: 444.55 g/mol
Other names:
- N-[2-[2-[2-(2-azidoethoxy)ethoxy]ethoxy]ethyl]biotinamide
- Biotin-PEG3-Azide
Fields of Interest: PEGylating reagent, organic chemistry, medicinal chemistry, click chemistry
Background & Applications:
Background
2-(2-(2-Azidoethoxy)ethoxy)-N-methylethanamine (CAS 875770-34-6) is a heterobifunctional azide-functionalized PEG linker based on a PEG2 backbone, featuring a terminal azide group and a secondary amine. The polyethylene glycol spacer provides hydrophilicity, flexibility, and compatibility with aqueous and biological systems while maintaining a compact linker length. The azide functionality enables efficient bioorthogonal click chemistry, including CuAAC and strain-promoted azide–alkyne cycloaddition. The secondary amine offers controlled reactivity toward activated carboxylic acids, NHS esters, isocyanates, and related electrophiles. This compound is a versatile intermediate within a broader portfolio of functionalized PEGs designed for predictable molecular modification.
Applications
2-(2-(2-Azidoethoxy)ethoxy)-N-methylethanamine, also known as Biotin-PEG3-Azide, is commonly used in bioconjugation, drug delivery, and materials science applications where short PEG spacing and orthogonal dual reactivity are required. Typical uses include stepwise construction of multifunctional linkers, PEGylation of small molecules and polymers, and surface or nanoparticle modification via click chemistry followed by amine coupling. As part of a comprehensive functionalized PEG product line, this azido–secondary amine PEG supports modular design strategies for pharmaceutical research, diagnostics, biomaterials, and advanced materials development.
Appearance: White solid
Purity: 99%
Storage: 0-3 °C for long term storage
Solubility: DMF
Literature:
- Organic Chemistry Frontiers, 2025, vol. 12, # 6, p. 2018 – 2024
- Journal of Materials Chemistry C, 2025, vol. 13, # 13, p. 6614 – 6623