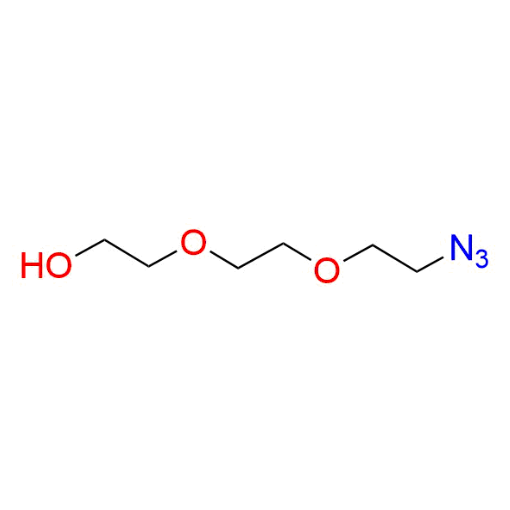
Name: 2-(2-(2-Azidoethoxy)ethoxy) ethanol (97% purity)
Molecular Formula: C6H13N3O4
CAS#: 86520-52-7
SMILES: OCCOCCOCCN=[N+]=[N-]
MDL#: MFCD11041099
Catalog#: AMTGC412-AE18
Molecular weight: 175.19 g/mol
Other names:
- triethylene glycol monoazide
- 2-(2-(2-azidoethoxy)ethoxy)ethan-1-ol
- azido-PEG3-OH or Azido-PEG3-alcohol
Fields of Interest: PEGylating reagent, Organic Synthesis, Medicinal Chemistry
Background & Applications:
Background
2-(2-(2-Azidoethoxy)ethoxy)ethanol (CAS 86520-52-7) is a monofunctional azide-functionalized PEG derivative based on a PEG2 backbone, featuring a terminal azide group and a hydroxyl group at the opposite end. The polyethylene glycol chain provides hydrophilicity, flexibility, and compatibility with aqueous and biological systems. The azide functionality enables efficient bioorthogonal click chemistry, including CuAAC and strain-promoted azide–alkyne cycloaddition, while the hydroxyl group offers additional derivatization options or can remain unreactive for controlled, single-point conjugation. This compound serves as a versatile intermediate within a broad portfolio of functionalized PEGs for precise molecular modification.
Applications
2-(2-(2-Azidoethoxy)ethoxy)ethanol is commonly used in bioconjugation, surface modification, and materials science applications where monofunctional azide reactivity and short PEG spacing are desired. Typical uses include click-based attachment of PEG chains to small molecules, polymers, and surfaces to improve solubility and reduce non-specific interactions, as well as preparation of intermediates for diagnostics, imaging agents, and advanced materials. As part of a comprehensive functionalized PEG product line, this azido PEG alcohol supports modular design strategies for pharmaceutical research, biomaterials development, and specialty chemical synthesis.
Appearance: Pale yellow liquid
Purity: 97%
Storage: 0-3 °C for long term storage
Solubility: Water, Chloroform, Ethyl Acetate, Ethyl Ether
Literature:
- European Journal of Medicinal Chemistry, 2026, vol. 303, art. no. 118430
- Journal of Organic Chemistry, 2025, vol. 90, # 1, p. 919 – 924