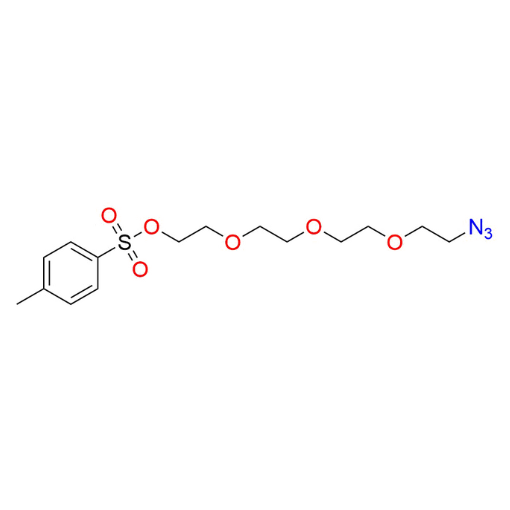
Name: 2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethyl 4-methylbenzenesulfonate (99%)
Molecular Formula: C15H23N3O6S
CAS#: 168640-82-2
SMILES: O=S(OCCOCCOCCOCCN=[N+]=[N-])(C1=CC=C(C)C=C1)=O
MDL#: MFCD22683295
Catalog#: AMTGC1279-AT24
Molecular weight: 373.42 g/mol
Other names:
- Azido-PEG4-Ots
- 11-azido-3,6,9-trioxa-undecanyl p-toluenesulfonate
- tetraethylene glycol p-toluenesulfonate azide
Fields of Interest: PEGylation, linker synthesis, polymer modification, materials science, click chemistry
Background & Applications:
Background
2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethyl 4-methylbenzenesulfonate (CAS 168640-82-2) is an activated azide-functionalized PEG intermediate based on a PEG4 backbone, featuring a terminal azide group and a tosylate (p-toluenesulfonate) leaving group. The polyethylene glycol spacer provides hydrophilicity, flexibility, and compatibility with aqueous and organic systems, while the tosylate enables efficient nucleophilic substitution to introduce a wide range of functional groups at the PEG terminus. The azide functionality supports bioorthogonal click chemistry, including CuAAC and strain-promoted azide–alkyne cycloaddition, making this compound a versatile building block within a comprehensive portfolio of functionalized PEGs for modular synthesis.
Applications
This azido PEG tosylate is commonly used in linker synthesis, polymer modification, and materials science applications where a reactive leaving group is needed for downstream functionalization. Typical uses include conversion to azido-PEG amines, thiols, halides, or other substituted PEG derivatives, as well as preparation of multifunctional intermediates for bioconjugation and drug delivery workflows. As part of a robust functionalized PEG product line, 2-(2-(2-(2-azidoethoxy)ethoxy)ethoxy)ethyl 4-methylbenzenesulfonate supports scalable, stepwise construction of tailored PEG linkers for pharmaceutical research, diagnostics, and advanced biomaterials development.
Appearance: Colorless liquid
Purity: 99%
Storage: 0-3 °C for long term storage
Solubility: DCM, Chloroform, EtOAc
Literature:
- Journal of Organic Chemistry, 2025, vol. 90, # 1, p. 919 – 924
- ACS Medicinal Chemistry Letters, 2023, vol. 14, # 1, p. 92 – 102