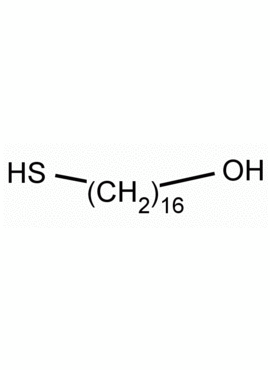
16-Mercaptohexadecan-1-ol, 16-Mercapto-1-hexadecanol CAS: 114896-32-1 MDL: MFCD03701687
Molecular weight: 274.51 g/mol
Molecular Formula: C16H34OS
CAS Number: 114896-32-1
Storage: Store at 2-8 Co, under dry conditions.
Synonyms: 16-Mercapto-1-hexadecanol, 114896-32-1, 16-sulfanylhexadecan-1-ol, 16-MERCAPTOHEXADECAN-1-OL, 1-Hexadecanol,16-mercapto-, 1-Hexadecanol, 16-mercapto-, 16-mercaptohexadecanol, ACMC-20dt2t, 16-Mercaptohexadecan-l-ol, SCHEMBL944745
Uses: Synthesis building block, Organic Synthesis, gold and self-assembled monolayers, nanolithography, polyfunctional monolayers, gold colloids
16-Mercaptohexadecan-1-ol is a synthetic specialty chemical useful in the synthesis of pharmaceuticals, nanocomposites, monolayer assemblies and specialty organic chemicals.
Selected references:
1.) Aizenberg, Joanna, Andrew J. Black, and George M. Whitesides. Oriented Growth of Calcite Controlled by Self-Assembled Monolayers of Functionalized Alkanethiols Supported on Gold and Silver. Journal of the American Chemical Society 121, no. (1999): 4500–4509. https://doi.org/10.1021/JA984254K.
2.) Aizenberg, Joanna, Paul V. Braun, and Pierre. Wiltzius. Patterned Colloidal Deposition Controlled by Electrostatic and Capillary Forces. Physical Review Letters 84, no. (2000): 2997–3000. https://doi.org/10.1103/PhysRevLett.84.2997.
3.) Bain, Colin D., E. Barry Troughton, Yu Tai Tao, Joseph Evall, George M. Whitesides, and Ralph G. Nuzzo. Formation of Monolayer Films by the Spontaneous Assembly of Organic Thiols from Solution onto Gold. Journal of the American Chemical Society 111, no. (1989): 321–35. https://doi.org/10.1021/ja00183a049.
4.) Banerjee, Sarbajit, and Stanislaus S. Wong. Synthesis and Characterization of Carbon Nanotube-Nanocrystal Heterostructures. Nano Letters 2, no. (2002): 195–200. https://doi.org/10.1021/nl015651n.
5.) Bao, Jie, Wei Chen, Taotao Liu, Yulin Zhu, Peiyuan Jin, Leyu Wang, Junfeng Liu, Yongge Wei, and Yadong. Li. Bifunctional Au-Fe3O4 Nanoparticles for Protein Separation. ACS Nano 1, no. (2007): 293–98. https://doi.org/10.1021/nn700189h.
6.) Creager, Stephen, C. J. Yu, Cindy Bamdad, Steve O’Connor, Tanya MacLean, Eric Lam, Yoochul Chong, et al. Electron Transfer at Electrodes through Conjugated ‘Molecular Wire’ Bridges. Journal of the American Chemical Society 121, no. (1999): 1059–64. https://doi.org/10.1021/JA983204C.
7.) Gole, Anand, and Catherine J. Murphy. Polyelectrolyte-Coated Gold Nanorods: Synthesis, Characterization and Immobilization. Chemistry of Materials 17, no. (2005): 1325–30. https://doi.org/10.1021/cm048297d.
8.) Gu, Hongwei, Zhimou Yang, Jinhao Gao, C. K. Chang, and Bing. Xu. Heterodimers of Nanoparticles: Formation at a Liquid-Liquid Interface and Particle-Specific Surface Modification by Functional Molecules. Journal of the American Chemical Society 127, no. (2005): 34–35. https://doi.org/10.1021/ja045220h.
9.) Hermes, Stephan, Felicitas Schroeder, Rolf Chelmowski, Christof Woell, and Roland A. Fischer. Selective Nucleation and Growth of Metal-Organic Open Framework Thin Films on Patterned COOH/CF3-Terminated Self-Assembled Monolayers on Au(111). Journal of the American Chemical Society 127, no. (2005): 13744–45. https://doi.org/10.1021/ja053523l.
10.) Huo, Fengwei, Zijian Zheng, Gengfeng Zheng, Louise R. Giam, Hua Zhang, and Chad A. Mirkin. Polymer Pen Lithography. Science (Washington, DC, United States) 321, no. (2008): 1658–60. https://doi.org/10.1126/science.1162193.
11.) Jung, Linda S., Charles T. Campbell, Timothy M. Chinowsky, Mimi N. Mar, and Sinclair S. Yee. Quantitative Interpretation of the Response of Surface Plasmon Resonance Sensors to Adsorbed Films. Langmuir 14, no. (1998): 5636–48. https://doi.org/10.1021/LA971228B.
12.) Kumar, Amit, Hans A. Biebuyck, and George M. Whitesides. Patterning Self-Assembled Monolayers: Applications in Materials Science. Langmuir 10, no. (1994): 1498–1511. https://doi.org/10.1021/la00017a030.
13.) Lahann, Joerg, Samir Mitragotri, Thanh-Nga Tran, Hiroki Kaido, Jagannathan Sundaram, Insung S. Choi, Saskia Hoffer, Gabor A. Somorjai, and Robert. Langer. A Reversibly Switching Surface. Science (Washington, DC, United States) 299, no. (2003): 371–74. https://doi.org/10.1126/science.1078933.
14.) Lee, Ki-Bum, So-Jung Park, Chad A. Mirkin, Jennifer C. Smith, and Milan. Mrksich. Protein Nanoarrays Generated by Dip-Pen Nanolithography. Science (Washington, DC, United States) 295, no. (2002): 1702–5. https://doi.org/10.1126/science.1067172.
15.) Nuzzo, Ralph G., Lawrence H. Dubois, and David L. Allara. Fundamental Studies of Microscopic Wetting on Organic Surfaces. 1. Formation and Structural Characterization of a Self-Consistent Series of Polyfunctional Organic Monolayers. Journal of the American Chemical Society 112, no. (1990): 558–69. https://doi.org/10.1021/ja00158a012.
16.) Ostuni, Emanuele, Robert G. Chapman, R. Erik Holmlin, Shuichi Takayama, and George M. Whitesides. A Survey of Structure-Property Relationships of Surfaces That Resist the Adsorption of Protein. Langmuir 17, no. (2001): 5605–20. https://doi.org/10.1021/la010384m.
17.) Piner, Richard D., Jin Zhu, Feng Xu, Seunghun Hong, and Chad A. Mirkin. ‘Dip-Pen’ Nanolithography. Science (Washington, D. C.) 283, no. (1999): 661–63. https://doi.org/10.1126/science.283.5402.661.
18.) Song, Shihua, Rose A. Clark, Edmond F. Bowden, and Michael J. Tarlov. Characterization of Cytochrome c/Alkanethiolate Structures Prepared by Self-Assembly on Gold. Journal of Physical Chemistry 97, no. (1993): 6564–72. https://doi.org/10.1021/j100126a037.
19.) Weisbecker, Carl S., Margaret V. Merritt, and George M. Whitesides. Molecular Self-Assembly of Aliphatic Thiols on Gold Colloids. Langmuir 12, no. (1996): 3763–72. https://doi.org/10.1021/LA950776R.
20.) Xu, Chenjie, Keming Xu, Hongwei Gu, Xiaofen Zhong, Zhihong Guo, Rongkun Zheng, Xixiang Zhang, and Bing. Xu. Nitrilotriacetic Acid-Modified Magnetic Nanoparticles as a General Agent to Bind Histidine-Tagged Proteins. Journal of the American Chemical Society 126, no. (2004): 3392–93. https://doi.org/10.1021/ja031776d.